Abstract
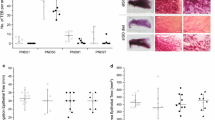
Isoflavone (ISO) exposure during adolescence has been demonstrated to modulate the estrogenic and proliferative sensitivity of the adult breast tissue. In this study, we investigated whether ISO exposure restricted to the period of puberty is sufficient to result in similar effects. Female rats were divided into three groups receiving either lifelong an ISO-free diet (IDD) or an ISO-rich diet (ISD), or an ISD from postnatal day (PND) 30 to PND 60 covering the time period of puberty (pISD). Serum concentrations of ISO and metabolites were determined at PND 50 and 80. At PND 50, ISD animals had significant higher equol serum levels than pISD animals. Onset of puberty occurred significantly earlier in the pISD and ISD group compared to the animals fed IDD. Cycle length was shortest in pISD group. To determine estrogen sensitivity of the adult breast tissue, adult rats were ovariectomized and subcutaneously treated either with estradiol (E2) or with genistein (GEN) for 3 days (PND 77–80). Analysis of Ki-67 and proliferating cell nuclear antigen (PCNA) expression showed a reduced proliferative response of the breast to E2 in pISD and ISD animals compared to IDD group, while the induction of progesterone receptor (PR) was higher in both IDD and pISD compared to ISD fed rats. Our results demonstrate that ISO exposure during puberty is sufficient to reduce the proliferative response of the adult mammary gland but not to reduce the response of classical E2 sensitive genes like the PR. In summary, our results demonstrate that animals exposed during different periods of their adolescence to ISO differ in several physiological aspects. In addition, the detected differences in the serum equol levels between pISD and ISD rats and the detected differences in the estrogen sensitivity of the breast clearly underline this assumption.




Similar content being viewed by others
Abbreviations
- DAI:
-
Daidzein
- ER:
-
Estrogen receptor
- E2 :
-
Estradiol
- GEN:
-
Genistein
- IDD:
-
Isoflavone-free diet
- ISD:
-
Isoflavone-standard diet
- ISO:
-
Soy isoflavone
- OVX:
-
Ovariectomized control group
- PCNA:
-
Proliferating cell nuclear antigen
- pISD:
-
Pre- to postpubertal ISD
- PND:
-
Postnatal day
- PR:
-
Progesterone receptor
- s.c.:
-
Subcutaneously
- TEB:
-
Terminal end bud
References
Atkinson C, Frankenfeld CL, Lampe JW (2005) Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 230(3):155–170
Barker DJ (2004) The developmental origins of chronic adult disease. Acta Paediatr Suppl 93(446):26–33
Cabanes A, Wang M, Olivo S et al (2004) Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis 25(5):741–748
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res 57(22):4987–4991
Cotroneo MS, Wang J, Fritz WA, Eltoum IE, Lamartiniere CA (2002) Genistein action in the prepubertal mammary gland in a chemoprevention model. Carcinogenesis 23(9):1467–1474
Diel P, Schmidt S, Vollmer G (2002) In vivo test systems for the quantitative and qualitative analysis of the biological activity of phytoestrogens. J Chromatogr B Anal Technol Biomed Life Sci 777(1–2):191–202
Dolinoy DC, Huang D, Jirtle RL (2007) Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 104(32):13056–13061
Dong JY, Qin LQ (2011) Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat 125(2):315–323
Fritz WA, Coward L, Wang J, Lamartiniere CA (1998) Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis 19(12):2151–2158
Gluckman PD, Hanson MA, Beedle AS (2007) Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol 19(1):1–19
Gu L, House SE, Prior RL et al (2006) Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr 136(5):1215–1221
Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ (2008) Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol 8:17
Hartman J, Edvardsson K, Lindberg K et al (2009) Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res 69(15):6100–6106
Hedlund TE, Maroni PD, Ferucci PG et al (2005) Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J Nutr 135(6):1400–1406
Heldring N, Pike A, Andersson S et al (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87(3):905–931
Hertrampf T, Schmidt S, Laudenbach-Leschowsky U, Seibel J, Diel P (2005) Tissue-specific modulation of cyclooxygenase-2 (Cox-2) expression in the uterus and the v. cava by estrogens and phytoestrogens. Mol Cell Endocrinol 243(1–2):51–57
Hulka BS, Moorman PG (2001) Breast cancer: hormones and other risk factors. Maturitas 38(1):103–13; discussion 113-6
Jackson MD, McFarlane-Anderson ND, Simon GA, Bennett FI, Walker SP (2010) Urinary phytoestrogens and risk of prostate cancer in Jamaican men. Cancer Causes Control 21(12):2249–2257
Janning P, Schuhmacher US, Upmeier A et al (2000) Toxicokinetics of the phytoestrogen daidzein in female DA/Han rats. Arch Toxicol 74(8):421–430
Ju YH, Allred KF, Allred CD, Helferich WG (2006) Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis 27(6):1292–1299
King RA (1998) Daidzein conjugates are more bioavailable than genistein conjugates in rats. Am J Clin Nutr 68(6 Suppl):1496S–1499S
Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE (2002) The specific role of isoflavones on estrogen metabolism in premenopausal women. Cancer 94(4):1166–1174
Lamartiniere CA (2002) Timing of exposure and mammary cancer risk. J Mammary Gland Biol Neoplasia 7(1):67–76
Lee SA, Shu XO, Li H et al (2009) Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr 89(6):1920–1926
Li Y, Liu L, Andrews LG, Tollefsbol TO (2009) Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer 125(2):286–296
Limer JL, Speirs V (2004) Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res 6(3):119–127
Lu LJ, Anderson KE, Grady JJ, Nagamani M (1996) Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev 5(1):63–70
Magee PJ, Rowland I (2012) Soy products in the management of breast cancer. Curr Opin Clin Nutr Metab Care 15(6):586–591
Messina M, Hilakivi-Clarke L (2009) Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer 61(6):792–798
Molinie B, Georgel P (2009) Genetic and epigenetic regulations of prostate cancer by genistein. Drug News Perspect 22(5):247–254
Moller FJ, Diel P, Zierau O, Hertrampf T, Maass J, Vollmer G (2010) Long-term dietary isoflavone exposure enhances estrogen sensitivity of rat uterine responsiveness mediated through estrogen receptor alpha. Toxicol Lett 196(3):142–153
Molzberger AF, Vollmer G, Hertrampf T, Moller FJ, Kulling S, Diel P (2012) In utero and postnatal exposure to isoflavones results in a reduced responsivity of the mammary gland towards estradiol. Mol Nutr Food Res 56(3):399–409
Mortensen A, Kulling SE, Schwartz H et al (2009) Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res 53(Suppl 2):S266–S309
Nagata C (2010) Factors to Consider in the Association Between Soy Isoflavone Intake and Breast Cancer Risk. J Epidemiol 20(2):83–89
Nechuta SJ, Caan BJ, Chen WY et al (2012) Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr 96(1):123–132
Odum J, Lefevre PA, Tittensor S et al (1997) The rodent uterotrophic assay: critical protocol features, studies with nonyl phenols, and comparison with a yeast estrogenicity assay. Regul Toxicol Pharmacol 25(2):176–188
Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S (2003) Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene 22(32):5011–5020
Padilla-Banks E, Jefferson WN, Newbold RR (2006) Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology 147(10):4871–4882
Penza M, Montani C, Romani A et al (2007) Genistein accumulates in body depots and is mobilized during fasting, reaching estrogenic levels in serum that counter the hormonal actions of estradiol and organochlorines. Toxicol Sci 97(2):299–307
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Reik W (2007) Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447(7143):425–432
Roger P, Daures JP, Maudelonde T et al (2000) Dissociated overexpression of cathepsin D and estrogen receptor alpha in preinvasive mammary tumors. Hum Pathol 31(5):593–600
Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA (2000) Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci USA 97(1):337–342
Savouret JF, Bailly A, Misrahi M et al (1991) Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J 10(7):1875–1883
Schwen RJ, Nguyen L, Plomley JB, Jackson RL (2012) Toxicokinetics and lack of uterotropic effect of orally administered S-equol. Food Chem Toxicol 50(5):1741–1748
Shoker BS, Jarvis C, Clarke RB et al (1999) Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol 155(6):1811–1815
Shu XO, Jin F, Dai Q et al (2001) Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev 10(5):483–488
Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA (2004) Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA 101(6):1566–1571
Toniolo PG, Levitz M, Zeleniuch-Jacquotte A et al (1995) A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst 87(3):190–197
Turner JV, Agatonovic-Kustrin S, Glass BD (2007) Molecular aspects of phytoestrogen selective binding at estrogen receptors. J Pharm Sci 96(8):1879–1885
Wang TT, Sathyamoorthy N, Phang JM (1996) Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis 17(2):271–275
Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L (2008) The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer 98(9):1485–1493
Whitten PL, Naftolin F (1992) Effects of a phytoestrogen diet on estrogen-dependent reproductive processes in immature female rats. Steroids 57(2):56–61
Wu AH, Ziegler RG, Horn-Ross PL et al (1996) Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev 5(11):901–906
Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC (2002) Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 23(9):1491–1496
Wu AH, Koh WP, Wang R, Lee HP, Yu MC (2008) Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer 99(1):196–200
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Molzberger, A.F., Soukup, S.T., Kulling, S.E. et al. Proliferative and estrogenic sensitivity of the mammary gland are modulated by isoflavones during distinct periods of adolescence. Arch Toxicol 87, 1129–1140 (2013). https://doi.org/10.1007/s00204-012-1009-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-012-1009-x