Abstract
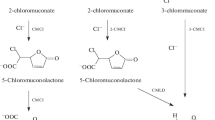
Xanthobacter flavus 14p1 used 1,4-dichlorobenzene as the sole source of carbon and energy but did not grow on other (chloro)aromatic compounds. 1,4-Dichlorobenzene was attacked by a chlorobenzene dioxygenase, and the intermediate chlorocatechol was metabolized by the modified ortho pathway. All enzymes necessary to convert 1,4-dichlorobenzene to 3-oxoadipate showed a low substrate specificity and also accepted the respective intermediates of chlorobenzene or 1,3-dichlorobenzene degradation. Of the three compounds chlorobenzene, 1,4-dichlorobenzene, and 1,3-dichlorobenzene, the latter was the most toxic for X. flavus 14p1. Furthermore, 1,3-dichlorobenzene did not induce chlorocatechol 1,2-dioxygenase activity of the organism. Chlorobenzene, however, induced chlorocatechol 1,2-dioxygenase, dienelactone hydrolase, and maleylacetate reductase activities. As demonstrated by chloride release, also chlorobenzene dioxygenase, chlorobenzene cis-dihydrodiol dehydrogenase, and chloromuconate cycloisomerase activities were present in chlorobenzene-induced cells, but chlorobenzene failed to support growth. Presumably a toxic compound was formed from one of the intermediates.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 10 June 1996 / Revision received: 23 December 1996 / Accepted: 18 January 1997
Rights and permissions
About this article
Cite this article
Sommer, C., Görisch, H. Enzymology of the degradation of (di)chlorobenzenes by Xanthobacter flavus 14p1. Arch Microbiol 167, 384–391 (1997). https://doi.org/10.1007/s002030050459
Issue Date:
DOI: https://doi.org/10.1007/s002030050459