Abstract
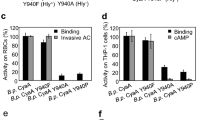
The recombinant Bordetella pertussis CyaA pore-forming (CyaA-PF) fragment was previously shown to be expressed separately in Escherichia coli as a soluble precursor that can be in vivo palmitoylated to exert haemolytic activity. In this study, PCR-based mutagenesis was employed to investigate the contributions to haemolysis of five predicted helices within the N-terminal hydrophobic region of the CyaA-PF fragment. Single proline substitutions were made for alanine near the centre of each predicted helix as a means of disrupting local secondary structure. All mutant proteins were over-expressed in E. coli as a 126-kDa soluble protein at levels comparable to the wild-type. Marked reductions in haemolytic activity against sheep erythrocytes of mutants, A510P, A538P, A583P and A687P pertaining to the putative helices 1500–522, 2529–550, 3571–593 and 5678–698, respectively, were observed. However, a slight decrease in haemolytic activity was found for the proline replacement in the predicted helix 4602–627 (A616P). MALDI–TOF–MS and LC–MS–MS analyses verified the palmitoylation at Lys983 of all five mutants as identical to that of the CyaA-PF wild-type protein, indicating that toxin modification via this acylation was not affected by the mutations. Altogether, these results suggest that structural integrity of the predicted helices 1, 2, 3 and 5, but not helix 4, is important for haemolytic activity, particularly for the putative transmembrane helices 2 and 3 that might conceivably be involved in pore formation of the CyaA-PF fragment.




Similar content being viewed by others
Abbreviations
- AC:
-
Adenylate cyclase
- CyaA:
-
Adenylate cyclase-haemolysin toxin
- CyaA-PF:
-
CyaA pore-forming
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- PCR:
-
Polymerase chain reaction
- PMSF:
-
Phenylmethylsulfonylfluoride
- RTX:
-
Repeat-in-toxin
- SDS-PAGE:
-
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
References
Angsuthanasombat C, Uawithya P, Leetachewa S, Pornwiroon W, Ounjai P, Kerdcharoen T, Kartzenmeier GR, Panyim S (2004) Bacillus thuringiensis Cry4A and Cry4B mosquito-larvicidal protein: homology based 3D model and implications for toxin activity. J Biochem Mol Biol 37:304–313
Barry EM, Weiss AA, Ehrmann IE, Gray MC, Hewlett EL, Goodwin MS (1991) Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J Bacteriol 173:720–726
Basler M, Masin J, Osicka R, Sebo P (2006) Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes. Infect Immun 74:2207–2214
Basler M, Knapp O, Masin J, Fiser R, Maier E, Benz R, Sebo P, Osicka R (2007) Segments crucial for membrane translocation and pore-forming activity of Bordetella adenylate cyclase toxin. J Biol Chem 282:12419–12429
Bauche C, Chenal A, Knapp O, Bodenreider C, Benz R, Chaffotte A, Ladant D (2006) Structural and functional characterization of an essential RTX subdomain of Bordetella pertussis adenylate cyclase toxin. J Biol Chem 281:16914–16926
Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A (1990) Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun 58:3242–3247
Benz R, Maier E, Ladant D, Ullmann A, Sebo P (1994) Adenylate cyclase toxin (CyaA) of Bordetella pertussis: Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J Biol Chem 269:27231–27239
Braun P, von Heijne G (1999) The aromatic residues Trp and Phe have different effects on the positioning of a transmembrane helix in the microsomal membrane. Biochemistry 38:9778–9882
Carbonetti NH, Artamonava GV, Andreasen C, Bushar N (2005) Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect Immun 73:2698–2703
Choe S, Bennett MJ, Fujii G, Curmi PM, Kantardjieff KA, Collier RJ, Eisenberg D (1992) The crystal structure of diphtheria toxin. Nature 357:216–222
Claros MG, von Heijne G (1994) TopPredII: an improved software for membrane protein structure predictions. Comput Appl Biosci 10:685–686
Dogovski C, Pi J, Pittard AJ (2003) Putative interhelical interactions within the PheP protein revealed by second-site suppressor analysis. J Bacteriol 185:6225–6232
Drechsler A, Potrich C, Sabo JK, Frisanco M, Guella G, Dalla Serra M, Anderluh G, Separovic F, Norton RS (2006) Structure and activity of the N-terminal region of the eukaryotic cytolysin equinatoxin II. Biochemistry 45:1818–1828
Ehrmann IE, Gray MC, Gordon VM, Gray LS, Hewlett EL (1991) Hemolytic activity of adenylate cyclase toxin from Bordetella pertussis. FEBS Lett 278:79–83
Ehrmann IE, Weiss AA, Goodwin MS, Gray MC, Barry E, Hewlett EL (1992) Enzymatic activity of adenylate cyclase toxin from Bordetella pertussis is not required for hemolysis. FEBS Lett 304:51–56
Eilers M, Shekar SC, Shieh T, Smith SO, Fleming PJ (2000) Internal packing of helical membrane proteins. Proc Natl Acad Sci USA 97:5796–5801
Fiser R, Masin J, Basler M, Krusek J, Spulakova V, Konopasek I, Sebo P (2006) Third activity of Bordetella adenylate cyclase (AC) toxin-hemolysin. Membrane translocation of AC domain polypeptide promotes calcium influx into CD11b+ monocytes independently of the catalytic and hemolytic activities. J Biol Chem 282:2808–2820
Glover T, Mitchell K (2002) Testing the difference between two means of independent samples. In: Glover T, Mitchell K (eds) An Introduction to Biostatistics. McGraw-Hill, New York, pp 163–168
Gueirard P, Druilhe A, Pretolani M, Guiso N (1998) Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun 66:1718–1725
Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardy-Castagnoli P, Guiso N, Ladant D, Leclerc C (2001) The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J Exp Med 193:1035–1044
Hackett M, Guo L, Shabanowitz J, Hunt DF, Hewlett EL (1994) Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science 266:433–435
Hewlett EL, Gordon VM, MaCaffery JD, Sutherland WM, Gray MC (1989) Adenylate cyclase toxin from Bordetella pertussis: identification and purification of the holotoxin molecule. J Biol Chem 264:19379–19384
Hofmann K, Stoffel W (1993) TMbase—a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler 374:166
Javadpour MM, Eilers M, Groesbeek M, Smith SO (1999) Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys J 77:1609–1618
Knapp O, Maier E, Polleichtner G, Masin J, Sebo P, Benz R (2003) Channel formation in model membranes by the adenylate cyclase toxin of Bordetella pertussis: effect of calcium. Biochemistry 42:8077–8084
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Malovrh P, Viero G, Serra MD, Podlesek Z, Lakey JH, Macek P, Menestrina G, Anderluh G (2003) A novel mechanism of pore formation: membrane penetration by the N-terminal amphipathic region of equinatoxin. J Biol Chem 278:22678–22685
Osickova A, Osicka R, Maier E, Benz R, Sebo P (1999) An amphipathic alpha-helix including glutamates 509 and 516 is crucial for membrane translocation of adenylate cyclase toxin and modulates formation and cation selectivity of its membrane channels. J Biol Chem 274:37644–37650
Parker MW, Feil SC (2005) Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol 88:91–142
Parker MW, Pattus F, Tucker AD, Tsernoglou D (1989) Structure of the membrane-pore-forming fragment of colicin A. Nature 337:93–96
Powthongchin B, Angsuthanasombat C (2008) High level of soluble expression in Escherichia coli and characterisation of the CyaA-pore forming fragment from a Bordetella pertussis Thai clinical isolate. Arch Microbiol 189:169–174
Rose T, Sebo P, Bellalou J, Ladant D (1995) Interaction of calcium with Bordetella pertussis adenylate cyclase toxin. Characterization of multiple calcium-binding sites and calcium-induced conformational changes. J Biol Chem 270:26370–26376
Rost B, Fariselli P, Casadio R (1996) Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci 5:1704–1718
Russ WP, Engelman DM (2000) The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296:911–919
Sakamoto H, Bellalou J, Sebo P, Ladant D (1992) Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J Biol Chem 267:13598–13602
Sebo P, Ladant D (1993) Repeat sequences in the Bordetella pertussis adenylate cyclase toxin can be recognized as alternative carboxy-proximal secretion signals by the Escherichia coli alpha-haemolysin translocator. Mol Microbiol 9:999–1009
Tilley SJ, Saibil HR (2006) The mechanism of pore formation by bacterial toxins. Curr Opin Struct Biol 16:230–236
Tusnády GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850
Vojtova J, Kofronova O, Sebo P, Benada O (2006) Bordetella adenylate cyclase toxin induces a cascade of morphological changes of sheep erythrocytes and localizes into clusters in erythrocyte membranes. Microsc Res Tech 69:119–129
van der Wel PCA, Strandberg E, Killian JA, Koeppe RE (2002) Geometry and intrinsic tilt of a tryptophan-anchored transmembrane alpha-helix determined by (2)H NMR. Biophys J 83:1479–1488
Welch RA (1991) Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol 5:521–528
Acknowledgments
We thank the Proteomic service center, Bio Service Unit, BIOTEC, Thailand for MALDI–TOF–MS and LC–MS–MS analyses. B.P. was financially supported in part by Faculty of Graduate Studies, Mahidol University and Faculty of Pharmacy, Silpakorn University. This work was funded by the Thailand Research Fund in cooperation with the Commission of Higher Education (to C.A.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Axel Brakhage.
Rights and permissions
About this article
Cite this article
Powthongchin, B., Angsuthanasombat, C. Effects on haemolytic activity of single proline substitutions in the Bordetella pertussis CyaA pore-forming fragment. Arch Microbiol 191, 1–9 (2009). https://doi.org/10.1007/s00203-008-0421-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-008-0421-3