Abstract
Aim: To evaluate the dietary calcium and vitamin D status in south Indian postmenopausal women. Methods: Postmenopausal women (n=164) were evaluated for their daily dietary calcium intake, phytate to calcium ratio, and bone mineral parameters. Their serum leutinizing hormone (LH), follicle-stimulating hormone (FSH), 25-hydroxyvitamin D (25[OH]D), and parathyroid hormone levels (PTH) were measured. Results: Their age and BMI were 59.5 ± 8 years and 27 ± 5 kg/m2, respectively. Their daily dietary intake of calcium was 323 ± 66 mg/day; phytate to calcium ratio, 0.56±0.1; LH, 26 ± 13.5 µIU/l; and FSH, 62.6 ± 30 µIU/l. Their dietary intake of calcium was low compared with the recommended daily/dietary allowance (RDA) of the Indian Council of Medical Research (ICMR) for the Indian population. Of the 164 patients studied, based on population-based reference values, 126 (77%) had normal 25(OH)D levels (9–37.6 ng/ml), and 38 (23%) had 25(OH)D deficiency. Using functional health-based reference values, 30 (18%) patients had normal 25(OH)D levels (>20 ng/ml), 85 (52%) had 25(OH)D insufficiency (10–20 ng/ml), and 49(30%) had 25(OH)D deficiency (<10 ng/ml). PTH and serum alkaline phosphatase (SAP) was significantly high in patients with 25(OH)D deficiency (p<0.05) compared with those with normal 25(OH)D levels. There was a negative correlation between 25(OH)D and PTH (r=−0.2; p<0.007) and SAP (r=−0.2; p<0.001). Dietary calcium correlated positively with dietary phosphates (r=0.8; p<0.001) and phytate to calcium ratio (r=0.75; p<0.001). Conclusions: Population-based reference values underdiagnosed vitamin D insufficiency and overdiagnosed normal vitamin D status. The diet was insufficient in calcium and high in phytate. About 82% of the study group had varying degrees of low 25-hydroxyvitamin D levels. The quality of diet has to be improved with enrichment/supplementation of calcium and vitamin D to suppress secondary hyperparathyroidism-induced bone loss and risk of fractures.
Similar content being viewed by others
Introduction
Serum 25-hydroxyvitamin D (25[OH]D) is the most reliable indicator of the vitamin D status of an individual. Vitamin D status of an individual is an estimation of vitamin D stores. In the past, measurement of serum 25(OH)D was limited by methodological differences [1]. With the evolution of 125I radioimmunoassay (RIA) for the estimation of serum 25(OH)D [2], the methodological differences have been overcome permitting an interlaboratory comparison of the values. However, improper definition of vitamin D deficiency and hypovitaminosis D makes meaningful comparison of the published literature very difficult. In clinical chemistry departments, the normal range for most of the analytes measured is derived from the values found in 95% of the population. It is not possible to establish such reference data for 25(OH)D. Since 25(OH)D levels are subject to variations in lifestyle and environmental characteristics (dress code, latitude and altitude, sun exposure, skin pigmentation, climate, policies of fortification and supplementation, nature of food stuffs rich in vitamin D, etc.) the normative data varies between laboratories [3, 4, 5, 6, 7]. These genuine geographic variations in calcium homeostasis restrict the locally estimated reference range to be used across countries. A locally developed set of population-based reference values could result in a person becoming 25(OH)D deficient en route from one country to another. Hence it is no longer appropriate to analyze 25(OH)D levels with respect to ranges supplied by the manufacturer.
It is essential to set criteria for 25(OH)D deficiency and insufficiency, so that means to exceed this level can be established (by monitored food fortification program). Setting thresholds for varying degrees of 25(OH)D insufficiency addresses the issue of 25(OH)D deficiency [8, 9, 10]. The term vitamin D deficiency is used for 25(OH)D values less than 10 ng/ml responsible for impaired mineralization. The term vitamin D insufficiency is used for 25(OH)D levels between 10 and 20 ng/ml responsible for parathyroid activity but without mineralization impairment [8, 9, 10, 11, 12].
Vitamin D insufficiency is associated with secondary hyperparathyroidism (SHPT), which can exacerbate osteoporosis by increasing mobilization of mineral and matrix from the skeleton [13, 14]. The increased bone turnover and decreased bone density leads to increased risk of fractures [11, 15, 16]. Estrogen deficiency in postmenopausal women is associated with increased bone remodeling. Vitamin D insufficiency and inadequate dietary calcium intake leads to further decrease in bone mass and risk of fracture. It is necessary to identify this subset of the population to design and target interventions to reduce the morbid consequences of low 25(OH)D levels. There is a lack of reliable epidemiological data of vitamin D status in postmenopausal Indian women. The present study evaluates the dietary calcium intake and vitamin D status in south Indian postmenopausal women and discusses its implications.
Methods
One hundred and sixty-four postmenopausal women were studied at Tirupati (lat 13.40° N, long 77.2° E), Chitoor district, Andhra Pradesh, from January 2000 to January 2001 for their physical characteristics, dietary habits, and bone mineral parameters. The average duration of sunlight in this region is around 8 to10 h per day in both summer and winter. The minimum and maximum temperatures of this region range from 17°C to 30°C in winter and 27°C to 39°C in summer. Often there is little seasonal variation in the peak sunlight. (This attenuates the seasonal variation in 25[OH]D.) The skin complexion of the subjects studied was wheatish to dark in color. The subjects are exposed to sunlight for a period of 4 to 6 h a day. Their exposure to sunlight was not restricted by clothing or a veil.
In all the patients, dietary intake of calcium, phosphorus, and phytates were documented by the dietary recall method. From the raw weight of the food consumed, the calcium and phosphorus intakes were calculated based on published food tables detailing the nutritive value of Indian foods [17]. About 10 ml of blood was collected from the most accessible peripheral vein between 0800 to 0900 hours in fasting state without using a tourniquet, for measurement of serum calcium, phosphorus, alkaline phosphatase, creatinine, and albumin concentrations. Blood was collected in test tubes kept under ice, for estimation of serum parathyroid hormone–intact molecule (PTH-intact) and 25(OH)D. Serum was separated in a refrigerated centrifuge at 4°C and stored at −20°C until analysis.
The levels of serum calcium, phosphorus, albumin, and alkaline phosphatase (SAP) were estimated using an automated biochemical analyzer (Synchron CX9; Beckman, USA). Normal values for these variables at our laboratory were as follows: serum calcium, 8.5 to 10 mg/dl; serum phosphorus, 2.5 to 4.8 mg/dl; SAP, 40 to 90 IU/l; and serum albumin, 3 to 5.5 g/dl.
The 25(OH)D concentrations were measured by competitive radioimmunoassay (RIA; DiaSorin, Stillwater, MN, USA; catalogue no. 68100E). The minimum detectable limit of 25(OH)D assay was 1.5 ng/ml. Reference range given by the kit manufacturer was 9 to 37.6 ng/ml. PTH-intact was measured by immunoradiometric assay (IRMA) (DiaSorin, Stillwater, MN, USA; catalogue no. 26100). Minimum detectable limit of PTH assay was 0.7 pg/ml. The reference range provided by the manufacturer was 13 to 54 pg/ml.
Serum follicle-stimulating hormone (FSH) was measured by IRMA (FSH-CTK-4, catalogue no. P001907; DiaSorin). The minimum detectable limit of FSH assay was 0.2 IU/l (menopause range >30 IU/l). Serum leutinizing hormone (LH) was measured by RIA (RIAK 10; Baba Atomic Research Center, Mumbai, India). The minimum detectable limit of LH assay was 4 IU/l (menopause range >12 IU/l).
All patients were ascertained to be euthyroid by relevant hormonal (TSH, T4) evaluation. Patients with chronic liver disease, history of medication with corticosteroids, estrogens, raloxifene, drugs affecting metabolism of vitamin D, and recent calcium or vitamin D intake were excluded from the study.
Statistical methods
All statistical analysis was performed using the SPSS package (version 11.5). One-way analysis of variance (ANOVA) was used to estimate the differences among the study groups. Descriptive results are presented as mean ± SD. Probability values <0.05 were considered as significant.
Results
The descriptive statistics of the physical characteristics, dietary intake, and biochemical and hormonal parameters of the whole group are presented in Table 1.
The diet of the study group consisted of 2,200 KJ/day approximately. Carbohydrates 55%, proteins 10%, fat 10%, vegetables 10%, and milk and milk products 15% constituted their diet. The carbohydrate source was from cereals: rice 50%, wheat 25%, and ragi (Eleusine coracana) 25%. Vegetable source was amaranth leaves, cauliflowers, carrots, ladies fingers, other seasonal vegetables, and tubers. Animal source of protein was consumed once a week. There was no other source of calcium or any other mineral or vitamin D. Milk is not fortified with calcium or vitamin D in India. The dietary calcium intake was low compared with the recommended daily/dietary allowance (RDA) of the Indian Council of Medical Research (ICMR) for Indian population.
Based on the reference range for 25(OH)D levels (9 to 37.6 ng/ml) given by the kit manufacturer [18], of the 164 patients studied 126 (77%) of them had normal 25(OH)D levels (16.9 ± 5.9 ng/ml), and 38 patients (23%) had 25(OH)D deficiency (6.84 ± 1.86 ng/ml).
Serum 25(OH)D levels of the patients were categorized based on functional health–based values [11] (Table 2). They were categorized as (1) normal vitamin D, 25(OH)D greater than 20 ng/ml; (2) vitamin D insufficiency (hypovitaminosis D), 25(OH)D between 10 and 20 ng/ml; and (3) vitamin D deficiency, 25(OH)D less than 10 ng/ml (moderate vitamin D deficiency, 25(OH)D between 5 and 10 ng/ml, and severe vitamin D deficiency, 25(OH)D less than 5 ng/ml). Based on the above categorization, only 30 patients (18%) had normal vitamin D levels. Vitamin D insufficiency was found in 85 patients (52%) and vitamin D deficiency in 49 (30%) patients. (Moderate vitamin D deficiency was found in 43 patients [26%] and severe vitamin D deficiency in 6 patients [4%]).
Using the “population-based reference values” from the kit manufacturer, 77% of the study group was diagnosed as having “normal vitamin D levels.” Whereas using “health-based reference values,” 18% of the study group was diagnosed to have normal vitamin D levels.
PTH was significantly high in patients with severe vitamin D deficiency (46.97 ± 34 pg/ml; p<0.05) and moderate vitamin D deficiency (26.84 ± 14 pg/ml; p<0.05) compared with those who had normal vitamin D levels. SAP was significantly high in severe vitamin D–deficiency patients (153.2 ± 98 IU/l; p<0.05) and moderate vitamin D–deficiency patients (145.6 ± 73.1 IU/l; p<0.05) compared with patients who had normal vitamin D levels.
There was a positive correlation between dietary calcium and dietary phosphates (r=0.8; p<0.001) and dietary phytate (r=0.75; p<0.001) in the whole group. Dietary phosphates positively correlated with dietary phytate (r=0.7; p<0.001) and phytate to calcium ratio (r=0.75; p<0.001). SAP correlated positively with dietary calcium (r=0.6; p<0.001), dietary phosphorus (r=0.5; p<0.001), dietary phytate (r=0.7; p<0.001), and phytate to calcium ratio (r=0.4; p<0.001).
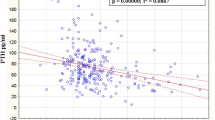
With linear regression analysis, a negative correlation was obtained between serum PTH and 25(OH)D (r=−0.2; p<0.007) (Fig. 1). There was a negative correlation between 25(OH)D and dietary calcium, phosphorus, phytate, and phytate to calcium ratio (r=−0.2; p<0.05) and SAP (r=−0.2; p<0.001). There was a negative correlation between PTH and serum calcium (r=−0.3; p<0.01).
Discussion
The best determinant of vitamin D status is the concentration of 25(OH)D levels in the serum because it is the major circulating metabolite that is directly dependent on substrate supply from both skin and oral sources. The definition of normal 25(OH)D levels based on functional health–based reference values has largely replaced the hitherto used reference values supplied by the kit manufacturer. It has the advantage of being clinically useful and is not subject to geographic or seasonal variations.
Classification of vitamin D status based on functional health based reference value defines “vitamin D deficiency as the critical level of 25(OH)D which prevents secondary hyperparathyroidism(SHPT).” This classification encompasses the vitamin D–calcium–PTH axis and its impact on bone [11, 19]. It is apt, based on scientific reasoning, and physiologically defines the vitamin D status of an individual.
In the present group, of the 126 patients (77%) diagnosed as having normal vitamin D status by population-based reference values, only 30 patients (18%) had normal vitamin D levels (25[OH]D, >20 ng/ml) and 85 patients (52%) had vitamin D insufficiency (25[OH]D, 10–20 ng/ml) when functional health–based reference values were used. Thus, population-based reference values underdiagnosed (failed to detect) vitamin D insufficiency and overdiagnosed the normal vitamin D status (Fig. 2).
It is axiomatic that any severity of deficiency of 25(OH)D deficiency must center on the relationship between PTH and 25(OH)D. Serum PTH negatively correlated (r=−0.2; p<0.007) with 25(OH)D levels in our study (Fig. 1). Most of the studies quoted in the literature have a similar negative correlation ranging from 0.2 to 0.3 [8, 11, 20, 21, 22, 23].
The diet was insufficient in calcium and high in phytate. The dietary calcium intake was low compared with the recommended daily/dietary allowance (RDA) of the Indian Council of Medical Research (ICMR) for the Indian population. The positive correlation between dietary calcium, phosphorus, and phytate points to the quality of diet consumed. There is no dietary source of vitamin D for these patients. It has been shown in the studies by Panwar et al. [24] that the calculated values for all nutrients are significantly higher than the analytical values. Hence, a patient with a calculated inadequate dietary intake of calcium in the background of high phytate, as in our study, may be far more calcium depleted than what is presumed to be! The inadequate dietary calcium intake is significant when viewed in the background of high phytate to calcium ratio associated with low 25(OH)D levels. Improving the quality of diet by reducing the phytate and enriching/supplementing with calcium will be of benefit for maintaining bone health. The negative correlation for 25(OH)D with PTH and SAP shows the internal consistency of the data.
Normal bone metabolism and bone integrity depend on appropriate repletion of vitamin D. Vitamin D insufficiency has adverse effects on bone mineral homeostasis. It affects calcium metabolism, osteoblastic activity, matrix ossification, bone mineral density (BMD), and bone remodeling. It is important to recognize 25(OH)D insufficiency in postmenopausal women, since vitamin D supplementation in these patients improves their BMD and prevents fracture. There is documentation to show that vitamin D and calcium supplementation is able to prevent hip fractures in elderly by normalizing the SHPT, reducing the bone remodeling, and stopping bone loss [20, 25, 26, 27, 28, 29].
Normal 25(OH)D levels are important for fully normal control of calcium absorption efficiency in the gut [21, 22]. It has been shown that the calcium absorption from the gut does not plateau until 25(OH)D exceeds 30 ng/ml [23]. Hypovitaminosis D impairs the calcium absorptive capacity of the gut. The accompanying SHPT leads on to accelerated bone remodeling [30, 31], decrease in bone mass, and increased risk of fracture [32, 33]. In cross-sectional studies, a positive relationship has been observed between 25(OH)D levels and BMD [34]. The Nottingham Neck of Femur (NoNoF) study has demonstrated a 68% prevalence of hypovitaminosis D in a population-based prospective cohort study of patients at risk for fractures [35]. In the present study, 82% of postmenopausal women have varying degrees of 25(OH)D low levels which are a modifiable risk factor for osteoporosis and easily correctable. Deficiency in 25(OH)D is the first step in the evolution of osteomalacia. Dietary calcium intake influences the progression and evolution (duration) of the disease. In situations of hypovitaminosis D associated with low dietary calcium intake in postmenopausal women, an imbalance in bone mineral homeostasis occurs when these groups of patients are exposed to factors that adversely affect vitamin D and bone mineral metabolism.
So far, vitamin D status in postmenopausal south Indian women has not been documented. Initial reports by the author and others from this country are in patients with primary hyperparathyroidism [36], healthy normal premenopausal women [37], in a group of patients attending rheumatology clinic [38], Indian paramilitary forces [39], and hospital staff [40]. The present study has clearly documented the insufficient dietary calcium intake in postmenopausal women with varying degrees of low vitamin D levels (in about 82% of the population). Low vitamin D impairs dietary calcium absorption and adversely affects bone mineralization which may be incorrectly seen as low BMD measurements.
Thus, recognizing hypovitaminosis D in postmenopausal women is important. Supplementing vitamin D and calcium suppresses the SHPT and reduces bone loss and risk of fracture. Several studies have documented that supplementing vitamin D and calcium has a synergistic effect in preventing proximal femoral fractures in postmenopausal and older patients [20, 25, 26, 41]. Hence, in south Indian postmenopausal women with vitamin D insufficiency, dietary enrichment/supplementation with calcium and vitamin D should be considered along with hormone replacement therapy. More multicenter studies are to be undertaken to document this problem in different parts of India.
References
Lips P, Chapuy MC, Dawson-Hughes B, Pols HAP, Holick MF (1999) International comparison of vitamin D measurements. Osteoporos Int 9:394–397
Hollis BW (2000) Comparison of commercially available125I-based RIA methods for determination of circulating 25-hydroxyvitamin D. Clin Chem 46:1657–1661
Webb AR, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D synthesis in human skin. J Clin Endocrinol Metab 67:373–378
Matsuoka LY, Worstman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF (1992) Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D. J Clin Endocrinol Metab 75:1099–1103
Clemens TL, Adams JS, Henderson SL, Holick MF (1982) Increased skin pigment reduces the capacity of skin to synthesis vitamin D3. Lancet 1:74–75
Need AG, Morris HA, Horowitz M, Nordin BEC (1993) Effects of skin thickness, age, body fat and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr 58:882–885
Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Marwer EB, Puliyel JM (2002) The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child 87:111–113
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S et al (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443
Dawson-Hughes B (1996) Calcium, vitamin D and vitamin D metabolites. In: Papapoulos SE, Lips P, Pols HAP, Hohnston CC, Delmas PD (eds) Osteoporosis. Proceedings of the 1996 world congress on osteoporosis. Elsevier International Congress Series, no. 118. Elsevier, Amsterdam, pp 229–303
Parfitt AM, Gallagher JC, Heaney RP, Johnston CC, Neer P, Whedon G (1982) Vitamin D and bone disease in the elderly. Am J Clin Nutr 32:1014–1031
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Harinarayan CV (2004) What’s in a name: 25(OH)D or 25(OH)D3? Natl Med J India 17(2):114–115
Luckert B, Higgins J, Stoskopf M (1992) Menopausal bone loss is partially regulated by dietary intake of vitamin D. Calcif Tissue Int 51:173–179
Villareal DT, Civitelli R, Chines A, Avioli LV (1991) Sub-clinical vitamin D deficiency in postmenopausal women with low vertebral bone mass. J Clin Endocrinol Metab 72:628–634
Holick MF (2000) Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 9:87–98
Harris SS, Soteriades E, Stina Coolidge JA, Mudgal S, Hughes BD (2001) Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 85:4125–4130
Gopalan C, Ramasastry BV, Balasubramanyam SC (1996) Nutritive value of Indian foods. National Institute of Nutrition, Hyderabad
Incstar (date unknown) Instruction manual: 25-hydroxyvitamin D125I RIA kit. Diasorin Incstar, Stillwater, MN, p 10
Parfitt AM, Mathews C, Rao D, Frame B, Kleerkoper M, Villanueava AR (1981) Impaired osteoblast function in metabolic bone disease. In: DeLuca HF, Frost HM, Jee WSS, Johnston CC, Parfitt AM (eds) Osteoporosis, recent advances in pathogenesis and treatment. University Park Press, Baltimore, pp 321–330
Chapuy MC, Chapuy P, Meunier PJ (1987) Calcium and vitamin D supplements: effects on calcium metabolism in elderly people. Am J Clin Nutr 46:324–328
Bischoff HA., Stahelin HB, Dick W, Akos R, Knecht M, Salis C et al (2003) Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Min Res 18:343–351
Heaney RP (2003) Vitamin D depletion and effective calcium absorption. A letter to the editor. J Bone Min Res18:1342
Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146
Panwar B, Punia D (2000) Analysis of composite diets of rural pregnant women and comparison with calculated values. Nutr Health 14:217–223
Chapuy MC, Arlot ME, Duboeuf F, Crouzet B, Delmas PD, Meunier PJ (1992) Vitamin D3 and calcium to prevent hip fractures in elderly women. N Eng J Med 327:1637–1642
Chapuy MC, Arlot ME, Delmas PD, Meunier PJ (1994). Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ 308:1081–1082
Dawson-Hughes B, Harris SS, Krall EA, Dallal GE (1997) Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Eng J Med 387:670–676
Chapuy MC, Pamphill R, Paris E, Kempf C, Schlichting M, Arnaud S, Garnero P, Meunier PJ (2002) Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int 18:257–264
Trivedi D P, Doll R, Khaw KT (2003) Effect of four monthly oral vitamin D3 (cholechaciferol) supplementation on fractures and mortality in men and women living in the community: double blind controlled trials. BMJ 386:469–472
Harinarayan CV, Gupta N, Kochupillai N (1995) Vitamin D status in primary hyperparathyroidism in India. Clin Endocrinol (Oxf) 43:351–358
Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N (2000) Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr 72:472–475
Joshi SR (1999) Hypovitaminosis D in patients attending a rheumatology clinic at a tertiary referral clinic. J Assoc Physicians India 47:463–464
Tandon N, Marwaha RK, Kalra S, Gupta N, Dudha A, Kochupillai N (2003) Bone mineral parameters in healthy young Indian adults with optimal vitamin D availability. Nat Med J India 16:298–302
Arya V, Bhambri R, Godbole MM, Mithal A (2004) Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporos Int 15(1):56–61
Prince RL, Dick I, Devine A, Price RI, Gutteridge DH, Kerr D et al (1995) The effect of menopause and age on calcitropic hormones: a cross sectional study of 655 healthy women aged 35 to 90. J Bone Miner Res 10:835–842
Brazier M, Kamel S, Maamer M, Agbomson F, Elesper I, Garabedian M et al (1995) The markers of bone remodeling in the elderly subjects: effects of vitamin D insufficiency and its correction. J Bone Miner Res 10:1753–1761
Greenspan SL, Martian LA, Myers ER, Krasnow MB, Kido TH (1994) Femoral bone loss progresses with age: a longitudinal study in women over age 65. J Bone Miner Res 9:1959–1965
Ensrud KE, Paiermo L, Black D, Cauley J, Jergas M, Orwoll ES et al (1995) Hip and calcaneal bone loss with advancing age: longitudinal results or the study of osteoporotic fractures. J Bone Miner Res 10:1778–1787
Mezquita-Raya P, Munoz-Torres M, Luna JDD, Luna V, Lopez-Rodriguez P, Torres-Vela E et al (2001) Relationship between vitamin D insufficiency, bone density and bone metabolism in healthy postmenopausal women. J Bone Miner Res 16:1408–1415
Sahota O, Gaynor K, Harwood RH, Hosking DJ (2001) Hypovitaminosis D and ‘functional hypoparathyroidism’: the NoNoF (Nottingham Neck of Femur) study. Age Ageing 30:467–472
Szulc P, Meunier PJ (2003) Synergistic effect of vitamin D and calcium in preventing proximal femoral fractures in older patients. Joint Bone Spine 70:157–160
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harinarayan, C.V. Prevalence of vitamin D insufficiency in postmenopausal south Indian women. Osteoporos Int 16, 397–402 (2005). https://doi.org/10.1007/s00198-004-1703-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-004-1703-5