Abstract
This systematic review and meta-analysis were conducted to compare the accuracy of component positioning, alignment and balancing techniques employed, patient-reported outcomes, and complications of robotic-arm assisted total knee arthroplasty (RATKA) with manual TKA (mTKA) and the associated learning curve. Searches of PubMed, Medline and Google Scholar were performed in October 2020 using PRISMA guidelines. Search terms included “robotic”, “knee” and “arthroplasty”. The criteria for inclusion were published clinical research articles reporting the learning curve for RATKA and those comparing the component position accuracy, alignment and balancing techniques, functional outcomes, or complications with mTKA. There were 198 articles identified, following full text screening, 16 studies satisfied the inclusion criteria and reported the learning curve of rTKA (n=5), component positioning accuracy (n=6), alignment and balancing techniques (n=7), functional outcomes (n=7), or complications (n=5). Two studies reported the learning curve using CUSUM analysis to establish an inflexion point for proficiency which ranged from 7 to 11 cases and there was no learning curve for component positioning accuracy. The meta-analysis showed a significantly lower difference between planned component position and implanted component position, and the spread was narrower for RATKA compared with the mTKA group (Femur coronal: mean 1.31, 95% confidence interval (CI) 1.08–1.55, p<0.00001; Tibia coronal: mean 1.56, 95% CI 1.32–1.81, p<0.00001). Three studies reported using different alignment and balancing techniques between mTKA and RATKA, two studies used the same for both group and two studies did not state the methods used in their RATKA groups. RATKA resulted in better Knee Society Score compared to mTKA in the short-to-mid-term follow up (95%CI [− 1.23, − 0.51], p=0.004). There was no difference in arthrofibrosis, superficial and deep infection, wound dehiscence, or overall complication rates. RATKA demonstrated improved accuracy of component positioning and patient-reported outcomes. The learning curve of RATKA for operating time was between 7 and 11 cases. Future well-powered studies on RATKAs should report on the knee alignment and balancing techniques utilised to enable better comparisons on which techniques maximise patient outcomes.
Level of evidence III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robotic total knee arthroplasty (TKA) is associated with improved accuracy of prosthesis implantation, and may improve outcomes and implant survival [1, 4, 15, 16]. The MAKO robotic-arm assisted TKA (RATKA) system (Stryker, Kalamazoo, Michigan, USA), in contrast to other robotic systems, is a “semi-active” system that allows the surgeon to interact with the robot during bone preparation, implant alignment and balancing of the knee [11]. These are important surgeon-controlled variables that affect patient outcomes, implant stability and long-term survivorship [5, 45, 53].
Recent systematic reviews comparing robotic TKA with manual TKA (mTKA) have not reported the techniques used to align and balance the knee, which may influence the associated functional outcome and survival of the prosthesis [9]. Furthermore, the only systematic review by Batailler et al. to analyse RATKA in isolation, excluding active systems, did not undertake meta-analysis for the outcomes assessed [2]. Reviews by Batailler et al. and Kayani et al. critically assessed outcomes, but pooled analyses was not conducted and limits interpretation of their data [2, 17]. Cadaveric cases were also included in these reviews, which may not reflect clinical results. Four recent meta-analysis have analysed the effects of robotic TKA on accuracy and functional outcomes when compared to mTKA, but most of the studies included were “fully active” systems [1, 7, 39, 43]. Only seven (31%) of 22 studies reported by Agarwal et al., one (10%) of ten reported by Chin et al., seven of 18 (39%) reported by Onggo et al. and none of the of seven studies reported by Ren et al. were “semi-active” robotic-arm assisted TKA (RATKA) [1, 7, 39, 43]. Therefore, the advantages of RATKA compared to mTKA is not clear due to the heterogeneity of systems included in these studies. Mancino et al. compared the rate of complications, but again also included mostly active systems in their review, which have been associated with a greater rate of complications compared to semi-active systems [29, 40].
The authors hypothesized that RATKA improves accuracy and patient-reported outcomes and has a lower complication rate compared to mTKA. Therefore, this systematic review and meta-analysis was conducted to compare the accuracy of component positioning, alignment and balancing techniques employed, patient-reported outcomes and complications of RATKA with mTKA and the associated learning curve.
Methods
A search of Medline, PubMed and Google Scholar was performed in October 2020 in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement. The study was registered on the PROSPERO International prospective register of systematic reviews (ID no. CRD42020218706).
All identified article titles and abstracts were screened independently by three authors (JZ, WSN and NDC), with those meeting the inclusion criteria screened further by full-text review. On occasions when it was not clear from the abstract if studies were of relevance, the full text of the article was reviewed. Unanimous consensus was met on the inclusion of proposed studies for full text review amongst the authors (JZ, WSN and NDC). Full text studies were further evaluated against the inclusion and exclusion criteria. The reference lists of included studies were reviewed to ensure no other relevant studies were overlooked.
Search terms and criteria for inclusion
Search terms included (‘robot’ [All fields] OR ‘robotic’ [All fields] OR ‘robotic surgical procedure’ [MeSH terms] with all entry terms and ‘robotic arm assisted’[All fields]) and (‘total knee’ [MeSH terms], OR ‘arthroplasty, replacement, total knee’ [MeSH terms] OR ‘arthroplasty’[MeSH terms]). A search limit for articles published from 2000 to 2020 was applied. A single search of PubMed and Medline yielded 52 abstracts. Two searches of Google Scholar using the search terms (a) all in title: robot total knee and (b) all in title: robotic total knee yielded 146 articles in total. The criteria for inclusion were published clinical research articles studying robotic total knee arthroplasty and reporting on functional outcomes or patient satisfaction or accuracy of component positioning or learning curve or complications. Studies were excluded if they were case reports, review articles, conference abstracts, non-clinical studies or were not available in the English language. For the purposes of this review, there was a focus on “semi-active” robotic systems and, therefore, “fully active” robotic systems were excluded from analysis.
Data extraction
The included studies were evaluated for the authors, year of publication, title, where it was published, study design (prospective or retrospective), age of patients, number of patients, follow-up (if applicable), the type of implant used and depending on the aims for the study: patient satisfaction, functional outcome, component accuracy, alignment and balancing techniques, complications and learning curve. In addition, the main conclusion from each study was also recorded. If two studies reported on the same cohort of patients, only the latter more complete cohort would be included in the current analysis.
Outcome measures
The primary objectives were to report the learning curve, accuracy of component positioning, alignment and balancing techniques, functional outcomes and complications within the included studies. Secondary objectives included presenting the demographic data and implants used across the included articles.
Quality assessment
Using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, all included publications were reviewed independently for potential risk of bias by three authors (JZ, SN, NDC). The assessment tool uses 14 questions to enable allocation of a score to each article (poor, fair or good). If there was disagreement regarding the scoring of a study, consensus was met after discussion amongst both assessors.
Statistical analysis
Simple descriptive analysis was performed for (a) learning curve of robotic-arm assisted total knee arthroplasty and for studies comparing (b) the accuracy of component positioning, (c) alignment and balancing techniques, (d) patient reported functional outcomes and (e) complications between robotic-arm assisted and manual total knee arthroplasty. Data were extracted from studies comparing (a) the accuracy of component positioning, (b) patient-reported functional outcomes (Knee Society Scores (KSS), Western Ontario and McMaster University Osteoarthritis Index (WOMAC)) and (c) complications (Manipulation under anaesthesia (MUA), superficial and deep infections, and wound dehiscence) between RATKA and mTKA to enable meta-analysis to be undertaken for these outcomes. The manipulation under anaesthesia (MUA), superficial and deep infections, pin site fractures and wound dehiscence were statistically assessed using Peto and the odds ratio were presented as the effect measure. The accuracy of component positioning, KSS, and the WOMAC scores were assessed using inverse variance and the mean difference was presented as the effect measure. For each outcome variable, 95% confidence intervals were presented. Heterogeneity among the studies were assessed using the χ2 test and I2. A fixed effect model was applied when I2 < 50%, and a random effects model when I2 > 50%. A p value < 0.05 was considered statistically significant in cases in which trials have no event in one arm or another. The meta-analysis was conducted using Review Manager 5.2 (Cochrane Collaboration, Oxford, UK).
Results
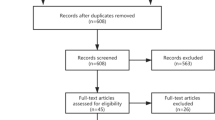
There were 198 articles identified in the initial search of databases and reference lists. After initial screening of titles and abstracts 31 articles met the inclusion criteria for review. On full text screening a further 13 studies were excluded from analysis: five represented articles assessing “fully active” robots [8, 13, 27, 46, 55]; six articles did not have a mTKA control group [21, 33, 37, 41, 48, 56]; four were non-clinical studies (Fig. 1) [12, 24, 35, 42]. A list of the 16 studies which met the inclusion criteria are illustrated in Table 1 [4, 18,19,20, 23, 25, 28, 30,31,32, 36, 44, 49,50,51,52]. Nine studies were identified from Medline and PubMed, and seven additional studies from Google Scholar (Table 1). The year of publication ranged from 2017 to 2020. Of the 16 published studies identified 11 were prospective and the remainder five were retrospective. There were no randomized controlled trials identified (Table 1, Fig. 2).
Learning curve (Level of evidence: good)
A total of five studies reported on the learning curve for RATKA (Table 2). All five studies assessed the learning curve for operating time which ranged from 7 to 80 cases [20, 32, 36, 44, 50]. Two studies utilised Cumulative Sum Control Chart (CUSUM) analysis to establish a learning curve inflexion point of proficiency (defined as the point of the learning curve where increasing operating times reverses) which ranged from 7 to 11 cases, dividing the learning curve for RATKA into two distinct phases, Phase 1 (initial learning segment) and Phase 2 (proficiency stage) [20, 44]. Marchand et al. observed continued decreases in mean operative times up to 1 year, after initial adaptation of robotics into workflows, with the mean operative time decreasing by 19 min, and only 12% of the cases exceeded 70 min operating time, quicker than mTKA performed by the same surgeons [32]. Cumulative robotic experience did not impact the accuracy of achieving the planned implant positioning, limb alignment, posterior condylar offset ratio, posterior tibial slope, or native joint line restoration [20]. This was quantified by Kayani et al. and affirmed in other studies which mainly reported no learning curve in accurate post-operative mechanical axis alignment [20, 32].
Component position accuracy (level of evidence: good)
There were six clinical studies identified that reported on accuracy of component positioning and all compared RATKA against mTKA (Table 3) [4, 20, 44, 50,51,52]. Three studies reported the posterior condylar offset ratio (PCOR) differences in RATKA and mTKA [20, 51, 52]. There was consistent evidence that RATKA resulted in significantly less differences between pre- and post-operative PCOR. Sultan et al. and Tucking et al. reported the lower differences between pre- and postoperative PCOR (p = 0.01 and p = 0.001 respectively) [51, 52]. When utilising RATKA, femoral components were placed with increased precision, within mean error range of 0.053 ± 0.020, as compared to 0.072 ± 0.035 when mTKA was utilised (Fig. 3d) [20, 51, 52]. Four studies reported the mechanical and coronal alignment, as well as sagittal alignment accuracies of implant positioning [20, 28, 36, 44]. When utilising RATKA, coronal alignment of the femur was within mean error range of 0.19 ± 1.14 while that for mTKA was 1.3 ± 1.34 (Fig. 3a). For tibia coronal alignment, it was 0.93 ± 1.57 for RATKA and 2.1 ± 1.76 for mTKA (Fig. 3b). As for posterior slope, it was 2.9 ± 1.59 for RATKA and 3.6 ± 2.51 for mTKA (Fig. 3c). These findings showed that RATKA was more precise compared to mTKA. The mTKAs were associated with wider range of component positioning, some of which are outside the preferred mean error range of ± 3 degrees. The pooled results from the forest plots demonstrated that component positioning using RATKA as compared to the mTKA was significantly more accurate (Femur coronal: mean difference 1.31, 95% confidence interval (CI) 1.08–1.55, p < 0.00001; Tibia coronal: mean difference 1.56, 95% confidence interval (CI) 1.32–1.81, p < 0.00001; Fig. 3a, b). In addition, Mahoney et al. showed that the femoral component external rotation with respect to the transepicondylar axis was more precise with the use of RATKA although this was not statistically significant (p = 0.195) [28].
Knee balancing and alignment techniques (level of evidence: fair)
Seven (44%) of the 16 clinical studies reported the balancing and/or the alignment techniques utilised for the TKAs groups (Table 4). Out of the seven studies, two stated that the mTKAs were performed using a standard measured resection technique followed by soft tissue releases to achieve a balanced and mechanically aligned knee, but did not define how the RATKA groups were balanced or how the alignment was achieved and whether it was using mechanical, kinematic or restricted kinematic alignment methods [30, 51]. Two studies used same methods in both groups, Bhimani et al. using gap balancing techniques and Mahoney et al. using measured resection techniques [4, 28]. The three remaining studies, however, used different methods for their RATKA and mTKA groups, namely kinematic alignment and gap balancing methods for RATKAs and measured resection methods for mTKAs [18, 20, 49].
Functional outcomes (level of evidence: good)
There were seven clinical studies reporting the functional outcomes following RATKA compared to mTKA (Table 5) [19, 23, 28, 30, 31, 35, 49]. Different outcome scores were utilised across the included studies, with the Knee Society Scores (KSS) being the most reported followed by Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores. Overall KSS ranged from 44.5 to 86.5-points and WOMAC scores ranged from 40 to 92-points in the RATKA group, whereas in mTKA group, it was 46.9 to 87.5-points for KSS scores and 16 to 86-points in WOMAC scores (Fig. 4). Khlopas et al. found evidence to suggest significant differences in the improvements made in scores but not in the absolute mean postoperative KSS scores achieved, while Mahoney et al. observed better scores in RATKA but it was not statistically significant (p = 0.159) [23, 28]. Meta-analysis of outcome data from the studies demonstrated RATKA resulted in a significantly better KSS score compared to mTKA in the short- to mid-term follow-up (mean difference 1.23, 95% CI 0.51–1.94, p = 0.004: Fig. 4). Meta-analysis of outcome data for WOMAC scores also demonstrated RATKA had significantly better scores compared to mTKA in the short- to mid-term follow-up (mean difference 3.72, 95% CI 1.72–5.72, p = 0.009: Fig. 4).
Complications (level of evidence: good)
Six studies reported on the complications between RATKA and mTKA groups (Table 6) [4, 19, 20, 25, 36, 49]. The most reported complications were arthrofibrosis requiring MUA, superficial or deep infections and wound dehiscence. Overall complication rates were low. No studies reported any pin site fractures or component revisions. In the RATKA group, arthrofibrosis rates ranged from 0 to 7.5%, superficial or deep infection rates ranged from 0 to 1.4% and wound dehiscence rates ranged from 0 to 2.5%. In mTKA groups arthrofibrosis rates ranged from 0 to 8.7%, superficial or deep infection rates ranged from 0 to 0.8%, and wound dehiscence rates ranged from 0 to 2.5%. A forest plot of pooled reported complication data demonstrated that there was no difference in arthrofibrosis, infection or wound dehiscence rates, but there was a higher risk (odds ratio 1.36, 95% confidence interval (CI) 0.63 to 2.94, p = 0.84; Fig. 5) for overall complication rate associated with mTKA compared to RATKA in short-term follow-up, but this was not significant.
Discussion
The key findings of this review were the following: (a) the learning curve of RATKA for surgical proficiency, stress and confidence levels was short (7–11 cases); (b) component positioning for RATKA was more precise when compared with mTKA; (c) short-term patient-reported outcomes were better with RATKA; (d) RATKA had similar complication rates as mTKA in the short-term and (e) there is a need for improved reporting of knee alignment and balancing techniques when comparing RATKA and mTKA.
The barrier for initial adoption is considered low for RATKA as the learning curve for RATKA was short. Decreasing operating times were noted after the first 7 to 11 cases, following initial phases of adaptation in techniques [20, 44]. This is coupled with the fact that there is no learning curve for accuracy of implant positioning. However, to achieve higher proficiencies, a relatively wider range in overall number of cases, 20 to 80, was required. The use of a defined methodology such as the CUSUM analysis is preferable as it identifies one exact turning point over a continuous curve, shown to be an accepted method in exploring the actual learning curve [20, 22, 54]. However, to gain mastery of RATKA techniques, with surgeons becoming more comfortable and experienced, it would be after about 80 cases, with reported consistently shorter operating times thereafter [32].
RATKAs have a theoretical advantage over mTKAs in component positioning in all three dimensions: coronal, sagittal and axial. The current analysis showed consistent evidence that RATKA resulted in less outliers in the component positions. This reflects the precision of the technique, irrespective of the knee alignment and balancing techniques used, which may differ from surgeon to surgeon. With the higher accuracy of component placement by RATKA, especially in the sagittal plane, the surgeon could potentially gap balance the knee more precisely with the use of RATKA compared to mTKA. Traditionally, balanced gaps have been considered a prerequisite for good function and endurance in TKA, and a balanced knee could affect long-term clinical outcomes [34, 47]. Furthermore, this precision and intra-operative feedback on the gap balancing could possibly minimise limitations in the conventional techniques and sizing options. Kayani et al. reported better early maximum knee flexion for RATKA 104.1 (90.0–120.0) degrees compared to mTKA 93.3 (90.0–110.0) degrees (p < 0.001), and there was a trend towards a reduced incidence of stiffness post-operatively (requiring MUA) for the RATKA cohort [19]. Future longer-term studies reporting the clinical outcomes of RATKAs should include such measures and evaluate the clinical relevance.
There is a need for improved reporting criteria for alignment and balancing techniques used in studies comparing RATKA with mTKA. Of the seven clinical studies that reported balancing and alignment techniques, only two studies used same balancing and alignment methods and two studies did not even state how their RATKAs groups were balanced or aligned [4, 28, 30, 51]. RATKA appear to be highly effective in improving the precision of restricted kinematic alignment techniques. Kayani et al. utilised measured resection for mTKA and restricted kinematic alignment for RATKA and recorded a significantly better component position accuracy in all three variables, namely coronal femur and tibia position and tibia posterior slope [20]. Mahoney et al. on the other hand utilised measured resection technique in both RATKA and mTKA, and better accuracies was shown only in tibia coronal positioning. However, due to inconsistent reporting methods in the stated technique for RATKA, there are currently insufficient data to indicate whether the restricted kinematic alignment technique yields better results for RATKAs. Overall, there is a need for improved reporting of the alignment and balancing techniques used (a critical aspect of comparing manual and robotic techniques). Future studies should report this to allow better conclusions to be made on the best alignment and balancing technique to be used with RATKA.
The current meta-analysis has demonstrated improved short-term patient-reported outcome measures (PROMs) for RATKA, compared to mTKA, according to the pooled KSS and WOMAC scores. Although there was a significant difference in the KSS of 1.23 points in favour of RATKA, this is not greater than the minimal clinical important difference (MCID) and, therefore, may not be clinically relevant [26]. Similarly, for WOMAC scores, there was a statistically significant difference but may not be clinically relevant [10]. Mahoney et al. reported on better physical scores of VR-12 in favour of RATKA, and again this was also not greater than the MCID [28, 38]. Although this may suggest that RATKA techniques may not make clinical differences in the short and medium term, it may also be reflective of the intrinsic limitations of the PROMs used, especially the ceiling effect. Scores such as the Forgotten joint score have a limited ceiling effect and may be better at demonstrating measurable clinically significant differences between RATKA and mTKA in future studies [11, 14].
Overall, the number of complications is low. Despite pooling together 1674 cases, only 24 arthrofibrosis requiring MUA, four infections, two wound dehiscence and no pin-site fractures were identified. The pooled results showed that the improved accuracy of component position obtained with RATKAs was not associated with any reduced joint stiffness nor was there increased risks of wound dehiscence or peri-prosthetic fractures. Given that only 33% of the weighted studies (n = 2/6) in the current analysis had a minimum follow-up of 1 year, of which only one had a follow-up of 15 months for the RATKA group, there remains a need for improved evidence with longer follow-up to better assess longer term complication and revision rates.
There are a few key limitations of the data set. First, the inclusion criteria, such as English language, may have excluded relevant studies. Second, the methodology has known limitations regarding the type of studies included (non-blinded, non-randomised prospective and retrospective cohort studies) and the difficulties in assessing the analyses without access to the raw data. Third, there was an important variability between the studies with respect to the type of outcome measurement used, the follow-up period and cohorts evaluated. Moreover, there are not yet any published randomized controlled trials. The studies on RATKAs are few and mainly have short-term follow-up. Future studies with longer term follow-up will be needed to provide more conclusive findings in assessing the outcomes and benefits. Another limitation was that two studies were excluded in the forest plot for functional outcomes of KSS scores because the spread of the data was not available for pooled analysis [36, 49]. Furthermore, the overall WOMAC score collected by Marchand et al. used a modified scale rather than the original WOMAC [30, 31]. These may have introduced bias into the analysis.
The main strength of this study, compared to previous systematic reviews, was the quantitative assessment of a singular image-based RATKA system. The assessments made with regard to the accuracy of component positioning in the various parameters allow the surgeon to be fully aware of the strengths and weaknesses of the system, modifying techniques to fine-tune bone resection, implant positioning and soft tissue releases to achieve the desired alignment and balancing, which was shown to be associated with improved functional outcomes.
Conclusion
RATKA demonstrated improved accuracy of component positioning and early patient-reported outcomes, though it may not be clinically significant. The learning curve of RATKA for operating time was between 7 and 11 cases. Future well-powered studies should report on the knee alignment and balancing techniques utilised in RATKAs to enable greater comparisons to be made on which techniques maximally benefit patient outcomes and provide better insights into alternate alignment philosophies.
Change history
18 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00167-021-06522-x
Abbreviations
- RATKA:
-
Robotic-arm assisted total knee arthroplasty
- mTKA:
-
Manual total knee arthroplasty
- CUSUM:
-
Cumulative sum control chart
- CI:
-
Confidence interval
- TKA:
-
Total knee arthroplasty
- PRISMA:
-
Preferred reporting items for systematic review and meta-analysis
- NIH:
-
National institutes of health
- KSS:
-
Knee society scores
- WOMAC:
-
Western Ontario and McMaster University Osteoarthritis Index
- MUA:
-
Manipulation under anaesthesia
- STAI:
-
Surrogate anxiety inventory
- PCOR:
-
Posterior condylar offset ratio
- PROMs:
-
Patient reported outcome measures
- MCID:
-
Minimal clinical important difference
References
Agarwal N, To K, McDonnell S, Khan W (2020) Clinical and radiological outcomes in robotic-assisted total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 35(11):3393-3409.e2
Batailler C, Fernandez A, Swan J, Servien E, Haddad FS, Catani F, Lustig S (2020) MAKO CT-based robotic arm-assisted system is a reliable procedure for total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-020-06283-z
Bautista M, Manrique J, Hozack WJ (2019) Robotics in total knee arthroplasty. J Knee Surg 32(7):600–606
Bhimani SJ, Bhimani R, Smith A, Eccles C, Smith L, Malkani A (2020) Robotic-assisted total knee arthroplasty demonstrates decreased postoperative pain and opioid usage compared to conventional total knee arthroplasty. Bone Jt Open 1(2):8–12
Boonen B, Schotanus MG, Kerens B, van der Weegen W, Hoekstra HJ, Kort NP (2016) No difference in clinical outcome between patient-matched positioning guides and conventional instrumented total knee arthroplasty two years post-operatively: a multicentre, double-blind, randomised controlled trial. Bone Joint J 98-B:939–944
Chen AF, Kazarian GS, Jessop GW, Makhdom A (2018) Robotic technology in orthopaedic surgery. J Bone Joint Surg Am 100(22):1984–1992
Chin BZ, Tan SSH, Chua KCX, Budiono GR, Syn NL, O’Neill GK (2020) Robot-assisted versus conventional total and unicompartmental knee arthroplasty: a meta-analysis of radiological and functional outcomes. J Knee Surg. https://doi.org/10.1055/s-0040-1701440
Cho KJ, Seon JK, Jang WY, Park CG, Song EK (2019) Robotic versus conventional primary total knee arthroplasty: clinical and radiological long-term results with a minimum follow-up of ten years. Int Orthop 43(6):1345–1354
Clement ND, Deehan DJ (2020) Minimum reporting criteria for robotic assisted total knee arthroplasty studies: alignment and balancing techniques should both be defined. Bone Joint Res 9(6):279–281
Clement ND, Bardett M, Weir D, Holland J, Gerrand C, Deehan DJ (2018) What is the minimum clinically important difference for WOMAC index after TKA? Clin Orthop Relat Res 476(10):2005–2014
Clement ND, Calliess T, Christen B, Deehan DJ (2020) An alternative technique of restricted kinematic alignment of the femur and gap balanced alignment of the tibia using computer aided navigation. Bone Joint Res 9(6):282–284
Cool CL, Jacofsky DJ, Seeger KA, Sodhi N, Mont MA (2019) A 90-day episode-of-care cost analysis of robotic-arm assisted total knee arthroplasty. J Comp Eff Res 8(5):327–336
Figueroa F, Wakelin E, Twiggs J, Fritsch B (2019) Comparison between navigated reported position and postoperative computed tomography to evaluate accuracy in a robotic navigation system in total knee arthroplasty. Knee 26(4):869–875
Hamilton DF, Loth FL, Giesinger JM, Giesinger K, MacDonald DJ, Patton JT, Simpson AH, Howie CR (2017) Validation of the English language Forgotten Joint Score-12 as an outcome measure for total hip and knee arthroplasty in a British population. Bone Joint J 99-B(2):218–224
Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL (2001) The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg 6(6):329–339
Jenkins PJ, Clement ND, Hamilton DF, Gaston P, Patton JT, Howie CR (2013) Predicting the cost-effectiveness of total hip and knee replacement: a health economic analysis. Bone Joint J 95-B(1):115–121
Kayani B, Konan S, Ayuob A, Onochie E, Al-Jabri T, Haddad FS (2019) Robotic technology in total knee arthroplasty: a systematic review. EFORT Open Rev 4(10):611–617
Kayani B, Konan S, Pietrzak JRT, Haddad FS (2018) Iatrogenic bone and soft tissue trauma in robotic-arm assisted total knee arthroplasty compared with conventional jig-based total knee arthroplasty: a prospective cohort study and validation of a new classification system. J Arthroplasty 33(8):2496–2501
Kayani B, Konan S, Tahmassebi J, Pietrzak JRT, Haddad FS (2018) Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty. Bone Joint J 100-B:930–937
Kayani B, Konan S, Huq SS, Tahmassebi J, Haddad FS (2019) Robotic-arm assisted total knee arthroplasty has a learning curve of seven cases for integration into the surgical workflow but no learning curve effect for accuracy of implant positioning. Knee Surg Sports Traumatol Arthrosc 27(4):1132–1141
Kayani B, Konan S, Ahmed SS, Chang JS, Ayuob A, Haddad FS (2020) The effect of anterior cruciate ligament resection on knee biomechanics. Bone Joint J 102-B(4):442–448
Kayani B, Konan S, Pietrzak JRT, Huq SS, Tahmassebi J, Haddad FS (2018) The learning curve associated with robotic-arm assisted unicompartmental knee arthroplasty: a prospective cohort study. Bone Joint J 100-B(8):1033–1042
Khlopas A, Sodhi N, Hozack WJ, Chen AF, Mahoney OM, Kinsey T, Orozco F, Mont MA (2020) Patient-reported functional and satisfaction outcomes after robotic-arm-assisted total knee arthroplasty: early results of a prospective multicenter investigation. J Knee Surg 33(7):685–690
Khlopas A, Chughtai M, Hampp EL, Scholl LY, Prieto M, Chang TC, Abbasi A, Bhowmik-Stoker M, Otto J, Jacofsky DJ, Mont MA (2017) Robotic-arm assisted total knee arthroplasty demonstrated soft tissue protection. Surg Technol Int 30:441–446
King CA, Jordan M, Bradley AT, Wlodarski C, Tauchen A, Puri L (2020) Transitioning a practice to robotic total knee arthroplasty is correlated with favorable short-term clinical outcomes—a single surgeon experience. J Knee Surg. https://doi.org/10.1055/s-0040-1712984
Lee WC, Kwan YH, Chong HC, Yeo SJ (2017) The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Knee Surg Sports Traumatol Arthosc 25(11):3354–3359
Liow MHL, Goh GS, Wong MK, Chin PL, Tay DK, Yeo SJ (2017) Robotic-assisted total knee arthroplasty may lead to improvement in quality-of-life measures: a 2-year follow-up of a prospective randomized trial. Knee Surg Sports Traumatol Arthrosc 25(9):2942–2951
Mahoney O, Kinsey T, Sodhi N, Mont MA, Chen AF, Orozco F, Hozack W (2020) Improved component placement accuracy with robotic-arm assisted total knee arthroplasty. J Knee Surg. https://doi.org/10.1055/s-0040-1715571
Mancino F, Cacciola G, Malahias MA, De Filippis R, De Marco D, Di Matteo VAG, Sculco PK, Maccauro G, De Martino I (2020) What are the benefits of robotic-assisted total knee arthroplasty over conventional manual total knee arthroplasty? A systematic review of comparative studies. Orthop Rev (Pavia) 12(Suppl 1):8657
Marchand RC, Sodhi N, Khlopas A, Sultan AA, Harwin SF, Malkani AL, Mont MA (2017) Patient satisfaction outcomes after robotic arm-assisted total knee arthroplasty: a short-term evaluation. J Knee Surg 30(9):849–853
Marchand RC, Sodhi N, Anis HK, Ehiorobo J, Newman JM, Taylor K, Condrey C, Hepinstall MS, Mont MA (2019) One-year patient outcomes for robotic-arm-assisted versus manual total knee arthroplasty. J Knee Surg 32(11):1063–1068
Marchand KB, Ehiorobo J, Mathew KK, Marchand RC, Mont MA (2020) Learning curve of robotic-assisted total knee arthroplasty for a high-volume surgeon. J Knee Surg. https://doi.org/10.1055/s-0040-1715126
Marchand RC, Sodhi N, Khlopas A, Sultan AA, Higuera CA, Stearns KL, Mont MA (2018) Coronal correction for severe deformity using robotic-assisted total knee arthroplasty. J Knee Surg 31(1):2–5
Matsuda Y, Ishii Y, Noguchi H, Ishii R (2005) Varus-valgus balance and range of movement after total knee arthroplasty. J Bone Joint Surg Br 87(6):804–808
Mont MA, Cool C, Gregory D, Coppolecchia A, Sodhi N, Jacofsky DJ (2019) Health care utilization and payer cost analysis of robotic arm assisted total knee arthroplasty at 30, 60, and 90 days. J Knee Surg. https://doi.org/10.1055/s-0039-1695741
Naziri Q, Cusson BC, Chaudhri M, Shah NV, Sastry A (2019) Making the transition from traditional to robotic-arm assisted TKA: What to expect? A single-surgeon comparative-analysis of the first-40 consecutive cases. J Orthop 16(4):364–368
Nodzo SR, Staub TM, Jancuska JM, Cobler-Lichter MD, Boyle KK, Rachala S (2020) Flexion space balancing through component positioning and its relationship to traditional anatomic rotational landmarks in robotic total knee arthroplasty. J Arthroplasty 35(6):1569–1575
Okoroha KR, Lu Y, Nwachukwu BU, Beletsky A, Patel BH, Verma NN, Cole B, Forsythe B (2020) How should we define clinically significant improvement on patient-reported outcomes measurement information system test for patients undergoing knee meniscal surgery? Arthroscopy 36(1):241–250
Onggo JR, Onggo JD, De Steiger R, Hau R (2020) Robotic-assisted total knee arthroplasty is comparable to conventional total knee arthroplasty: a meta-analysis and systematic review. Arch Orthop Trauma Surg 140(10):1533–1549
Park SE, Lee CT (2007) Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty 22:1054–1059
Persohn SA, Paramasivam M, Zhang J, Bhowmik-Stoker M, Otto J, Wahdan A, Salem HS, Choplin RH, Territo PR, Mont MA (2020) A novel vector-based computer tomography alignment measurement protocol for total knee arthroplasty. Surg Technol Int 36:371–378
Pierce J, Needham K, Adams C, Coppolecchia A, Lavernia C (2020) Robotic arm-assisted knee surgery: an economic analysis. Am J Manag Care 26(7):e205–e210
Ren Y, Cao S, Wu J, Weng X, Feng B (2019) Efficacy and reliability of active robotic-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a systematic review and meta-analysis. Postgrad Med J 95(1121):125–133
Savov P, Tücking L-R, Windhagen H, Ettinger M (2020) Comparable surgery time for robotic assisted total knee arthroplasty after initial learning curve. Orthop J Sports Med. https://doi.org/10.1177/2325967120S00312
Scott CE, Oliver WM, MacDonald D, Wade FA, Moran M, Breusch SJ (2016) Predicting dissatisfaction following total knee arthroplasty in patients under 55 years of age. Bone Joint J 98-B:1625–1634
Shalhoub S, Lawrence JM, Keggi JM, Randall AL, DeClaire JH, Plaskos C (2019) Imageless, robotic-assisted total knee arthroplasty combined with a robotic tensioning system can help predict and achieve accurate postoperative ligament balance. Arthroplast Today 5(3):334–340
Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM (2002) Insall Award paper: Why are total knee arthroplasties failing today? Clin Orthop Relat Res 404:7–13
Sires JD, Craik JD, Wilson CJ (2019) Accuracy of bone resection in MAKO total knee robotic-assisted surgery. J Knee Surg. https://doi.org/10.1055/s-0039-1700570
Smith AF, Eccles CJ, Bhimani SJ, Denehy KM, Bhimani RB, Smith LS, Malkani AL (2019) Improved patient satisfaction following robotic-assisted total knee arthroplasty. J Knee Surg. https://doi.org/10.1055/s-0039-1700837
Sodhi N, Khlopas A, Piuzzi NS, Sultan AA, Marchand RC, Malkani AL, Mont MA (2018) The learning curve associated with robotic total knee arthroplasty. J Knee Surg 31(1):17–21
Sultan AA, Samuel LT, Khlopas A, Sodhi N, Bhowmik-Stoker M, Chen A, Orozco F, Kolisek F, Mahoney O, Smith L, Malkani A, Molloy RM, Mont MA (2019) Robotic-arm assisted total knee arthroplasty more accurately restored the posterior condylar offset ratio and the insall-salvati index compared to the manual technique; a cohort-matched study. Surg Technol Int 34:409–413
Tücking L-R, Savov P, Windhagen H, Ettinger M (2020) Posterior condylar offset ratio more precisely reconstructed with robotic arm-assisted total knee arthroplasty when compared to conventional manual technique. Orthop J Sports Med. https://doi.org/10.1177/2325967120S00324
Vince K (2016) Mid-flexion instability after total knee arthroplasty: woolly thinking or a real concern? Bone Joint J 98-B(1):84–88
Yap CH, Colson ME, Watters DA (2007) Cumulative sum techniques for surgeons: a brief review. ANZ J Surg 77(7):583–586
Yang HY, Seon JK, Shin YJ, Lim HA, Song EK (2017) Robotic total knee arthroplasty with a cruciate-retaining implant: a 10-year follow-up study. Clin Orthop Surg 9(2):169–176
Yeo JH, Seon JK, Lee DH, Song EK (2019) No difference in outcomes and gait analysis between mechanical and kinematic knee alignment methods using robotic total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 27(4):1142–1147
Acknowledgements
The authors acknowledge the kind assistance rendered by Michael A Mont in the conversion required for the WOMAC scores collected by his team into a percentage scale.
Funding
There was no funding received in the production of the manuscript.
Author information
Authors and Affiliations
Contributions
JZ: wrote the manuscript and collaborated with the team on the selection of articles for inclusion. WSN: collaborated with the team on the selecion of articles for inclusion. NN: performed the statistical analysis of the current meta-analysis. PG: guidance in the preparation of the manuscipt. PMS: guidance in the preparation of the manuscript. GJM: research idea creation, direction of the manuscript writing. JTP: research idea creation, direction of the manuscript writing. NDC: research idea creation, literature review and providing the mentorship for preparation and execution of the stages of the review and meta-analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the conflicts of interest as stated in the forms submitted.
Ethical approval
There is no need for ethical approvals for this systematic review and meta-analysis, and this study has been registered with PROSPERO International prospective register of systematic reviews (ID no. CRD42020218706).
Informed consent
There is no informed consent required in the preparation of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The correct version of abstract updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Ndou, W.S., Ng, N. et al. Robotic-arm assisted total knee arthroplasty is associated with improved accuracy and patient reported outcomes: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 30, 2677–2695 (2022). https://doi.org/10.1007/s00167-021-06464-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06464-4