Abstract
Purpose
Early prediction of extracorporeal membrane oxygenation (ECMO) requirement in term newborns with persistent pulmonary hypertension (PPHN), partially responding to inhaled nitric oxide (iNO) and/or high-frequency oscillatory ventilation (HFOV), based on oxygenation parameters.
Methods
This was a retrospective cohort study in 53 partial responders from among 133 term newborns with PPHN born between 2002 and 2007. Alveolar-to-arterial oxygen gradient (AaDO2) values were determined in these 53 partial responders during the initial 72 h of iNO and/or HFOV treatment and compared between newborns who ultimately did (n = 11) and did not (n = 42) need ECMO.
Results
Over 72 h, partial responders not requiring ECMO showed a more profound AaDO2 decrease than those who needed ECMO (median decline 242.5 mmHg, IQR 144 to 353 mmHg, vs. 35 mmHg, IQR −15 to 123 mmHg; p = 0.0007). A decline of <123 mmHg over 72 h predicted the need for ECMO (sensitivity 82 %, specificity 79 %). At 72 h, AaDO2 was significantly lower in partial responders without the need for ECMO than in those who did need ECMO (median 369 mmHg, IQR 258 to 478 mmHg, vs. 570 mmHg IQR 455 to 590 mmHg; p = 0.0008). An AaDO2 >561 mmHg at 72 h predicted the need for ECMO (sensitivity 64 %, specificity 95 %, positive predictive value 78 %).
Conclusions
In term newborns with PPHN partially responding to iNO and/or HFOV, oxygenation-based prediction of the need for ECMO appears to be possible after 72 h. ECMO centers are encouraged to develop their own prediction model in order to prevent both lung damage and unnecessary ECMO runs.
Similar content being viewed by others
Introduction
Newborns with persistent pulmonary hypertension (PPHN) have traditionally been treated with conventional mechanical ventilation, oxygen, sedation, nonselective vasodilators, inotropic and vasopressor support, muscle relaxants, and interventions to reverse acidosis. Extracorporeal membrane oxygenation (ECMO) has been used as rescue treatment whenever conservative management fails. Although new treatment modalities, such as high-frequency oscillatory ventilation (HFOV) and inhaled nitric oxide (iNO), have become available, traditional ECMO entry criteria—an oxygenation index (OI) of >40 or an alveolar-to-arterial oxygen gradient (AaDO2) of >600–610 mmHg for several hours—are still used [1–4]. Several studies have shown that iNO, especially in combination with HFOV, can significantly ameliorate oxygenation and reduce the need for ECMO treatment [5–17]. However, their use sometimes causes a delay in the initiation of ECMO. In newborns treated in this way, prolongation of iNO and/or HFOV with high oxygen levels may induce chronic lung disease (CLD) and extend the intensive care stay with all its associated risks [18]. On the other hand, initiating ECMO too early unnecessarily exposes newborns to harmful procedures (e.g. major vessel cannulation, systemic anticoagulation), while they could have been successfully managed with iNO, HFOV, and other noninvasive therapies [1].
Current ECMO entry criteria (Table 1) are suitable for situations in which newborns with PPHN respond well or not at all to iNO and/or HFOV treatment [1]. In newborns with a partial response to iNO and/or HFOV, adequate oxygenation can usually be maintained, but (short-term) reversal of PPHN does not occur. Because current ECMO criteria are never met in these latter newborns, they may develop lung damage and even die due to prolonged treatment with iNO and/or HFOV. Therefore, adapted ECMO entry criteria are needed for partial responders. So far, only Kössel et al. [19] have established guidelines that help predict whether a response to iNO and/or HFOV is temporary or persistent. These authors concluded that an OI of ≥25 after 72 h of iNO and/or HFOV predicts the need for ECMO in newborns with acute respiratory failure and signs of PPHN. We sought to determine whether the need for ECMO can be predicted in a timely manner in PPHN patients with an intermediate response to iNO and/or HFOV, using AaDO2 as oxygenation parameter.
Materials and methods
Patient selection and treatment
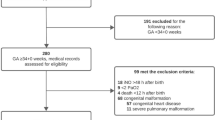
Newborns with a gestational age ≥37 weeks and a birth weight ≥2,000 g, born between 1 January 2002 and 31 December 2007, admitted to our department for treatment of PPHN using iNO and/or HFOV, with an AaDO2 ≥500 mmHg at the start of iNO and/or HFOV, were selected for this study. An AaDO2 ≥500 mmHg can be regarded as an indicator of severe PPHN [20]. The clinical diagnosis of PPHN was confirmed echocardiographically (tricuspid valve regurgitation, right-to-left or bidirectional shunt at the atrial or ductal level) in all patients. Newborns with (congenital) heart disease, pulmonary anomalies (including congenital diaphragmatic hernia, CDH), severe hypoxic-ischemic encephalopathy, major intracranial hemorrhage, and those that died ≤72 h after birth, were excluded (Fig. 1).
Flow chart. PDA patent ductus arteriosus, VSD ventricular septal defect, SPB-deficiency surfactant protein-B deficiency, CDH congenital diaphragmatic hernia, MAS meconium aspiration syndrome, CLD chronic lung disease, IC intensive care, MV mechanical ventilation, DOL day of life; aSevere allo-immune hemolytic anemia (2), pulmonary hemorrhage (1), asphyxia (1), respiratory distress syndrome (1); IC stay, MV and DOL are denoted as median days; ECMO run is in median hours
Treatment of PPHN was started with conventional ventilation (Babylog 8000; Dräger, Lübeck, Germany) with high fractions of inspired oxygen (FiO2) to maintain postductal arterial oxygen saturation ≥95 % and partial arterial oxygen pressure (paO2) >80 mmHg (10.6 kPa). Patients were sedated with morphine and/or midazolam and, if needed, given a muscle relaxant. If PPHN persisted, iNO (Solmix 1000; Dutch Technical Gas Company, Tilburg, The Netherlands) was added to a maximum of 20 ppm. In newborns with systemic hypotension, cardiovascular support consisted of dopamine (maximum 20 μg/kg/min), dobutamine (maximum 20 μg/kg/min), norepinephrine (maximum 1 μg/kg/min), corticosteroids, and/or epinephrine (maximum 1 μg/kg/min). Surfactant (beractant, Survanta; Ross Laboratories, Columbus, OH; one dose = 100 mg/kg) was administered to newborns with meconium aspiration syndrome (MAS). HFOV (Loudspeaker type, 3100A; SensorMedics Corporation, Yorba Linda, CA) was used when conventional ventilation failed to improve oxygenation and/or in the presence of severe hypercarbia or barotrauma.
Patients with PPHN resolution were gradually weaned from iNO and/or HFOV on the condition that paO2 remained >80 mmHg. In those without PPHN resolution, venovenous or venoarterial ECMO was started once they fulfilled current ECMO entry criteria (for conciseness referred to as ‘ECMO need’) and did not meet the exclusion criteria (Table 1).
Data collection and processing
The medical records of all newborns were reviewed. Sex, gestational age, birth weight, Apgar score after 1 and 5 min, and primary diagnosis were recorded. The following data were collected for 72 after the initiation of iNO and/or HFOV: ventilation mode, ventilator settings, mean doses of iNO, inotropic and vasopressor agents, and use of corticosteroids and surfactant. For each newborn, all arterial blood gases determined over 72 h were recorded. The FiO2 value at the time of each blood gas determination was retrieved from the patient’s record and, using the mean barometric pressure in the vicinity of our hospital on the day in question (www.knmi.nl/climatology/daily_data), the corresponding AaDO2 was calculated using the formula AaDO2 = (patm − 47) × FiO2 − (paO2 + paCO2). Because a high paCO2 falsely lowers AaDO2, a paCO2 of 37.5 mmHg (= 5 kPa) was used to calculate AaDO2 in patients with hypercarbia (paCO2 >6.5 kPa). Each patient had at least 6, but usually 12 to 24 blood gas determinations per day. Mean daily AaDO2 was determined for the first 72 h of iNO and/or HFOV or until ECMO was initiated. Outcome parameters were: days on mechanical ventilation, ECMO need, hours on ECMO, day of life on which ECMO was initiated, days on the NICU, CLD (FiO2 >0.21 beyond day 28), and survival.
We defined the following groups (Fig. 1): (1) responders (successfully managed with iNO and/or HFOV and both modalities ceased at ≤72 h); (2) nonresponders (failed to improve on iNO and/or HFOV and ECMO initiated at ≤72 h); (3) partial responders (improved to a certain extent, but iNO and/or HFOV still needed beyond 72 h). Partial responders were subdivided into two groups: one that did and one that did not eventually need ECMO. We determined whether AaDO2 at 24, 48 or 72 h after the start of iNO and/or HFOV could predict ECMO need in partial responders. Since paO2 and paCO2, and therefore AaDO2, can fluctuate from one hour to another, we also assessed whether prediction based on mean daily AaDO2 was more accurate. Finally, we determined whether the course of AaDO2 during 72 h of iNO and/or HFOV treatment was indicative of ECMO need.
Statistical analysis
Nominal data are presented as number with percentage, ordinal and continuous variables as median with interquartile range (IQR). Background characteristics, survival and CLD were compared using Fisher’s exact test. The Mann-Whitney U test was used for comparison of AaDO2 values, time on mechanical ventilation and days on the NICU. Receiver operating characteristic (ROC) curves were created to determine whether AaDO2 at t = 24, t = 48, or t = 72 h (or mean AaDO2 on day 1, 2 or 3) could be used as predictor of ECMO. Logistic regression analysis was used to identify cut-off values for AaDO2 that best distinguished partial responders who needed ECMO from those who did not. The statistical significance level was set at p < 0.05. All statistical analyses were performed using SPSS Software (SPSS, Chicago, IL).
Results
Between 2002 and 2007, 133 term newborns were treated at our center for PPHN with iNO and/or HFOV. After excluding 40 newborns, 93 were eligible for further analysis. Of these, 12 were classified as responders and 28 as nonresponders. The remaining 53 were partial responders, of whom 11 did and 42 did not need ECMO. Patient numbers, primary diagnoses, and outcomes for all groups are shown in Fig. 1. Background characteristics are shown in Table 2.
AaDO2 just before initiation of iNO and/or HFOV was significantly higher in partial responders who did not require ECMO than in those who did need ECMO (618 mmHg, IQR 604 to 632 mmHg vs. 595 mmHg, IQR 567 to 623 mmHg; p = 0.02). Partial responders needing ECMO received significantly more surfactant. All remaining background characteristics were comparable between the two partial responder groups, including the level of cardiovascular and ventilator support (Table 2). Only one patient (responder group) did not receive iNO.
In partial responders without ECMO need, AaDO2 showed a persistent decline from the initiation of iNO and/or HFOV onwards (Fig. 2). In this group, a median decrease of 242.5 mmHg (IQR 144 to 353 mmHg) occurred over 72 h. In partial responders with ECMO need, AaDO2 decreased in the first 48 h, but showed an increase thereafter. This group showed an overall decrease of only 35 mmHg (IQR −15 to 123 mmHg). This difference in decline over 72 h was significant (p = 0.0007). At t = 24 h and t = 48 h, median AaDO2 was not significantly different between the two partial responder groups. At t = 72 h, median AaDO2 was significantly lower in partial responders without ECMO need (369 mmHg, IQR 258 to 478 mmHg) than in partial responders requiring ECMO (570 mmHg, IQR 455 to 590 mmHg; p = 0.0008).
A ROC curve was plotted (not shown) for the median decline in AaDO2 over 72 h as a predictor of ECMO need. The area under this curve (AUC) was 0.83. Sensitivity and specificity combined were maximal (82 % and 79 %, respectively) at a median AaDO2 decline of 123 mmHg over 72 h.
Figure 3 shows that the ability of AaDO2 to predict ECMO need increased with time elapsed from the start of iNO and/or HFOV. At t = 72 h, the AUC was 0.83. An AaDO2 of >561 mmHg at 72 h had a sensitivity, specificity and positive predictive value (PPV) of 64 %, 95 %, and 78 %, respectively, for the prediction of ECMO need in partial responders.
In none of the statistical analyses did the use of mean daily AaDO2, instead of AaDO2 at t = 24, 48 and 72 h, result in more significant outcomes (results not shown). Survival was comparable between the two partial responder groups. Mortality was directly related to PPHN. CLD incidence was higher and time on mechanical ventilation and time on the NICU were significantly longer in partial responders with ECMO need (Fig. 1).
Discussion
Newborns with PPHN are commonly managed with iNO, HFOV and/or surfactant, only followed by ECMO when these modalities fail. Difficulty arises when newborns are partially responsive to iNO and/or HFOV to the extent that current ECMO criteria are not met, though aggressive respiratory and cardiovascular support remains necessary, which may result in death or the development of CLD. There is a need for adapted ECMO criteria that appropriately address this situation.
Several studies have attempted to predict ECMO need based on the initial response of newborns to either iNO or high-frequency ventilation. However, none of these studies specifically addressed the group of partial responders. Baumgart et al. [21] demonstrated that a significant decrease in OI (i.e. 15) after 6 h of high-frequency jet ventilation renders the need for ECMO unlikely. A similar conclusion was drawn by Paranka et al. [22], who found that an arterial-to-alveolar (a/A) oxygen ratio of ≤0.08 following 6 h of HFOV predicts ECMO need in newborns with acute respiratory failure and without CDH or lung hypoplasia with a sensitivity of 77 % and specificity of 92 %. These authors also ascertained that the a/A ratio just before initiation of HFOV and a diagnosis of CDH/lung hypoplasia are independent predictors of ECMO need.
Amongst the studies that used the short-term response to iNO as a predictor of ECMO need is that of Biban et al., who found that newborns showing a significant improvement in oxygenation parameters within 1 to 6 h from the start of iNO can usually be successfully managed with iNO in combination with conventional mechanical ventilation. Newborns showing no improvement in oxygenation during the first hours of iNO administration should be treated with ECMO immediately [12]. Fakioglu et al. [20] recommend initiating ECMO if OI or AaDO2 have not significantly decreased after 4 h of iNO. According to these authors, an OI of >20 or an AaDO2 of >600 mmHg after 4 h of iNO is associated with a relative risk for ECMO of 7 and 4.5, respectively. Since the response to iNO at 4 h was not indicative of ‘late ECMO requirement’ (i.e. ECMO after 2–4 days of iNO), these results do not apply to partial responders. A substitute marker for ‘late ECMO’ was nevertheless identified: OI at the start of iNO was significantly higher in patients who needed ECMO compared to those who could be managed with iNO alone (63.0 ± 27.0 vs. 31.4 ± 7.7, respectively). In newborns with MAS, Friedlich et al. [23] found that the OI after 6 h of iNO was significantly higher in those who required ECMO compared to those who did not (55.9 ± 40.1 vs. 29.9 ± 13.7, respectively). In contrast to the aforementioned studies, Goldman et al. [24] stressed that an early response to iNO does not guarantee a sustained effect, nor does it guarantee that ECMO can be avoided. The main disadvantage of all these studies is that none of them investigated the response to the combination of iNO and HFOV to develop a prediction model, despite the availability of evidence demonstrating that HFOV enhances alveolar recruitment and thereby favors iNO delivery, rendering the simultaneous use of these two modalities more effective than either alone [9, 13]. In addition, whereas short-term responses to either iNO or HFOV can be used to determine the need for early ECMO, they cannot be used to predict whether ECMO will be required after several days of iNO and/or HFOV.
The only study that has looked at the possibility of predicting ECMO need in newborns with PPHN after a longer period of iNO and/or HFOV is that of Kössel et al. [19]. In this well-designed, prospective trial, the OI after 24, 48 and 72 h of iNO and/or HFOV was used to predict whether a response would be temporary or sustained. All newborns who did not require ECMO showed a persistent decline in OI during the initial 72 h of iNO and/or HFOV treatment. The authors concluded that an OI of ≥25 is a useful entry criterion for ECMO in newborns with respiratory failure pretreated with iNO and/or HFOV for 72 h. An important shortcoming of this study is that its conclusions are based on small patient numbers. We therefore endeavored to confirm the findings of Kössel et al. in a larger group of patients. Furthermore, the group of patients having a ‘sustained response’ in the study of Kössel et al. included both responders and partial responders. We left responders out of our analysis to develop adapted ECMO criteria specifically addressing the group of newborns in which a decision regarding ECMO has to be made after 72 h of iNO and/or HFOV.
We found that a decline in AaDO2 of <123 mmHg during 72 h of iNO and/or HFOV treatment predicted ECMO need with a sensitivity and specificity of 82 % and 79 %, respectively. Although desirable, reasonably accurate prediction of ECMO need was not feasible before 72 h after the start of iNO and/or HFOV. An AaDO2 of >561 mmHg at 72 h predicted ECMO need with a sensitivity of 64 %, a specificity of 95 %, and a PPV of 78 %. Contrary to the results reported by Fakioglu et al. [20] and Paranka et al. [22], oxygenation parameters at baseline were not indicative of ultimate ECMO need in our study. Baseline AaDO2 was significantly higher in partial responders not requiring ECMO than in those ending up on ECMO. We do not have a clear explanation for this observation.
The results of our study are comparable to those of Kössel et al. [19]. We therefore believe that it is possible to predict ECMO need based on either OI or AaDO2 after 72 h of iNO and/or HFOV treatment. Although our overall survival was good (89 %), the high incidence of CLD (45 %) in partial responders started on ECMO after 8 days of iNO and/or HFOV supports our belief that morbidity may be reduced by earlier recognition of ECMO need. Due to limited patient numbers, it was impossible to perform subgroup analysis and develop adapted ECMO entry criteria for specific primary diagnoses. In general, newborns with respiratory distress syndrome are most likely to show a sustained response to iNO and HFOV, whereas patients with MAS or sepsis/pneumonia usually respond to a lesser extent [12, 19, 22]. The use of iNO in structural PPHN (e.g. CDH) is controversial [17] and the usefulness of HFOV in this situation is currently under investigation in a prospective trial [25].
Limitations
Our study has limitations inherent to retrospective cohort studies. Data were not gathered according to a predetermined study protocol, but acquired during everyday clinical practice. The facts that PPHN treatment is highly protocolized and ECMO criteria are strictly used in our center, render this limitation a little less restrictive. It should be noted, however, that we do not have specific criteria to initiate HFOV. We generally switch to HFOV in patients with severe hypercarbia or barotrauma or when conventional ventilation fails. In our center, we use AaDO2 as the oxygenation parameter instead of OI, because we want to avoid the high airway pressures required to achieve an OI of >40 [15, 26]. AaDO2 does not, however, reflect the intensity of ventilator support. In order to provide some insight into the intensity of mechanical ventilation, we determined the median mean airway pressure during the 72 h of iNO and/or HFOV treatment in all groups. Mean airway pressure was not significantly different between partial responders with and without ECMO need. Furthermore, the PPV of our adapted ECMO criteria was 78 %, which implies that one in every five newborns will be put on ECMO unnecessarily. However, one should bear in mind that the original ECMO entry criteria (AaDO2 >600 mmHg) have been used with a similar level of accuracy [4]. Finally, we acknowledge that the adapted ECMO criteria provided in this report can only be applied to newborns with PPHN treated in our own center and in centers with an approach very similar to ours.
Conclusions
In our population of term newborns with PPHN, an AaDO2 of >561 mmHg after 72 h of iNO and/or HFOV predicted eventual ECMO need. We recommend that other centers develop their own adapted ECMO criteria. Prospective studies should then be performed to validate these criteria and test their ability to prevent lung damage due to prolonged aggressive ventilation on the one hand and unnecessary ECMO runs on the other.
References
Bahrami KR, Van Meurs KP (2005) ECMO for neonatal respiratory failure. Semin Perinatol 29:15–23
Elbourne D, Field D, Mugford M (2002) Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev (1):CD001340
Ford JW (2006) Neonatal ECMO: current controversies and trends. Neonatal Netw 25:229–238
Beck R, Anderson KD, Pearson GD et al (1986) Criteria for extracorporeal membrane oxygenation in a population of infants with persistent pulmonary hypertension of the newborn. J Pediatr Surg 21:297–302
González A, Fabres J, D’Apremont I et al (2010) Randomized controlled trial of early compared with delayed use of inhaled nitric oxide in newborns with a moderate respiratory failure and pulmonary hypertension. J Perinatol 30:420–424
Dewhurst C, Ibrahim H, Göthberg S, Jónsson B, Subhedar N (2010) Use of inhaled nitric oxide in the newborn period: results from the European inhaled nitric oxide registry. Acta Paediatr 99:854–860
Konduri GG, Kim UO (2009) Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am 56:579–600
Hoffman GM, Ross GA, Day SE, Rice TB, Nelin LD (1997) Inhaled nitric oxide reduces utilization of extracorporeal membrane oxygenation in persistent pulmonary hypertension of the newborn. Crit Care Med 25:352–359
Kinsella JP, Truog WE, Walsh WF et al (1997) Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 131:55–62
The Neonatal Inhaled Nitric Oxide Study Group (1997) Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med 336:597–604
Roberts JD Jr, Fineman JR, Morin FC et al (1997) Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 336:605–610
Biban P, Trevisanuto D, Pettenazzo A, Ferrarese P, Baraldi E, Zacchello F (1998) Inhaled nitric oxide in hypoxaemic newborns who are candidates for extracorporeal life support. Eur Respir J 11:371–376
Kinsella JP, Abman SH (1998) High-frequency oscillatory ventilation augments the response to inhaled nitric oxide in persistent pulmonary hypertension of the newborn: nitric oxide study group. Chest 114(1 Suppl):100S
Clark RH, Kueser TJ, Walker MW et al (2000) Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med 342:469–474
Schaible T, Hermle D, Loersch F et al (2010) A 20-year experience on neonatal extracorporeal membrane oxygenation in a referral center. Intensive Care Med 36:1229–1234
Christou H, Van Marter LJ, Wessel DL et al (2000) Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med 28:3722–3727
Finer N, Barrington KJ (2006) Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 18(4):CD000399
Gill BS, Neville HL, Khan AM, Cox CS Jr, Lally KP (2002) Delayed institution of extracorporeal membrane oxygenation is associated with increased mortality rate and prolonged hospital stay. J Pediatr Surg 37:7–10
Kössel H, Bauer K, Kewitz G, Karaca S, Versmold H (2000) Do we need new indications for ECMO in neonates pretreated with high-frequency ventilation and/or inhaled nitric oxide? Intensive Care Med 26:1489–1495
Fakioglu H, Totapally BR, Torbati D et al (2005) Hypoxic respiratory failure in term newborns: clinical indicators for inhaled nitric oxide and extracorporeal membrane oxygenation therapy. J Crit Care 20:288–293
Baumgart S, Hirschl RB, Butler SZ, Coburn CE, Spitzer AR (1992) Diagnosis-related criteria in the consideration of extracorporeal membrane oxygenation in neonates previously treated with high-frequency jet ventilation. Pediatrics 89:491–494
Paranka MS, Clark RH, Yoder BA, Null DM Jr (1995) Predictors of failure of high-frequency oscillatory ventilation in term infants with severe respiratory failure. Pediatrics 95:400–404
Friedlich P, Noori S, Stein J et al (2005) Predictability model of the need for extracorporeal membrane oxygenation in neonates with meconium aspiration syndrome treated with inhaled nitric oxide. J Pediatr Surg 40:1090–1093
Goldman AP, Tasker RC, Haworth SG, Sigston PE, Macrae DJ (1996) Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 98:706–713
Van den Hout L, Tibboel D, Vijfhuize S, CDH-EURO Consortium et al (2011) The VICI-trial: high frequency oscillation versus conventional mechanical ventilation in newborns with congenital diaphragmatic hernia: an international multicentre randomized controlled trial. BMC Pediatr 11:98
Van Meurs KP, Hintz SR, Sheehan AM (2005) ECMO for neonatal respiratory failure. In: Meurs KP, Hally KP, Peek G, Zwischenberger JB (eds) Extracorporeal cardiopulmonary support in critical care, 3rd edn. Michigan, Ann Arbor, pp 273–295
Acknowledgments
We thank Mrs. N. Peer and Mr. W. Lemmens for performing the statistical analyses. We also thank the referring NICUs in The Netherlands for allowing us to gather our data at their institutions.
Conflicts of interest
The authors have no financial or ethical conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Berkel, S., Binkhorst, M., van Heijst, A.F.J. et al. Adapted ECMO criteria for newborns with persistent pulmonary hypertension after inhaled nitric oxide and/or high-frequency oscillatory ventilation. Intensive Care Med 39, 1113–1120 (2013). https://doi.org/10.1007/s00134-013-2907-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2907-y