Abstract
Objective
The aim of this study was to identify risk factors for redialysis in postoperative patients with acute renal failure (ARF) who had previously been weaned from acute dialysis. Although recovery of renal function is anticipated in patients with ARF, no data have been reported on successful weaning from acute dialysis.
Design and setting
Retrospective observational case-control study in a 64-bed surgical ICU.
Patients and methods
Success in discontinuing dialysis was defined as cessation from dialysis for at least 30 days. A total of 304 postoperative patients who underwent acute renal replacement therapy in a surgical ICU between July 2002 and April 2005 were included. SOFA score biochemical data and renal function parameters were assessed on the day after the last session of renal replacement therapy, designated as day 0 (D0).
Results
We could wean 94 patients (30.9%) from acute dialysis for more than 5 days, and 64 of these (21.1%) were successfully weaned for at least 30 days. The independent predictors for resuming dialysis within 30 days were: (a) longer duration of dialysis (OR 1.06), (b) higher SOFA score on D0 (OR 1.44), (c) oliguria (urine output < 100cc/8 h; OR 4.17) on D1, and (d) age over 65 years (OR 6.35). The area under the ROC curve was 0.880. Two-way analysis of variance with repeated measurements over time showed a larger decline in SOFA score and an increase in urine output in patients with successful cessation of dialysis. Kaplan–Meier analysis showed a significant difference in early resumption of dialysis between patients with or without oliguria at D0.
Conclusions
More than two-thirds of patients weaned from postoperative acute dialysis for more than 5 days were free of dialysis for at least 30 days. Less urine output, longer duration of dialysis, age over 65 years, and higher disease severity score are predictive of a patient's redialysis after initial weaning from acute dialysis.
Similar content being viewed by others
Introduction
Although recovery of renal function is usually expected in patients undergoing acute dialysis, data are limited regarding successful weaning from acute dialysis [1–3]. Physicians often confront the decision of continuing or withdrawing dialysis. Perioperative ischemic reperfusion injury may result in acute renal failure (ARF), from which patients can generally recover [4]. However, there remain a large number of patients whose kidneys fail to recover from ARF, and therefore long-term dialysis is required [5, 6]. Clinically many indicators of renal recovery from ARF have been proposed. For example, a nonoliguric state is usually considered an indicator of mild kidney injury and a good prognostic indicator in ARF [7] and may lead to withholding renal replacement therapy (RRT) in anticipation of recovery. Older age is a risk factor for renal failure in critically ill patients undergoing acute dialysis [3]. Shorter periods of dialysis have also been reported to predict independence from dialysis in ARF [8]. Nevertheless, the factors associated with the inability to withdraw acute RRT have not been elucidated. There is also a paucity of data on the course in patients weaned from acute RRT and subsequently relapsing and requiring RRT again.
The aim of the current study was to determine the indicators that predict the resumption of dialysis in patients with postoperative ARF who were initially weaned from acute RRT. We underscore the point that there is likely an under-appreciation of the severity of renal injury at time of cessation of dialysis and also highlight the need for more standardized criteria for withdrawing dialysis.
Patients and methods
Patients
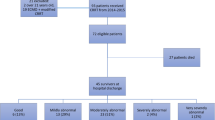
This retrospective observational case-control study was conducted in the 64-bed surgical ICU of a tertiary hospital, where 304 patients underwent acute RRT following major surgical procedures between July 2002 and January 2005. Surgical procedures were considered major if the length of the patient's hospital stay in a given diagnosis-related group exceeded 2 days [9, 10]. Exclusion criteria were: (a) patients who resumed dialysis within 5 days (fewer than three sessions during an alternative-day protocol of RRT) after attempting dialysis cessation (n = 42) or not weaned at all (n = 104), (b) patients who underwent renal transplantation (n = 5), (c) patients who were terminally ill and had stopped dialysis because of a do-not-resuscitate order (n = 45), (d) patients who only underwent acute RRT for less than 3 days (n = 25), and (e) patients who resumed dialysis because of reoperation (n = 9). A successful weaning was defined as the cessation of dialysis for at least 30 days.
The study thus included 94 postoperative patients (68 men, 26 women; mean age 58.8 ± 20.0 years) who were weaned from acute dialysis for more than 5 days and compared the patients (n = 64) who were successfully weaned from RRT for at least 30 days to those who were not (n = 40). The mean duration of RRT was 15.6 ± 14.7 days. Most patients (54.3%) received intermittent hemodialysis at cessation. Cardiovascular surgery was performed in 50 patients (53.2%), thoracic surgery in 5 (5.3%), neurological surgery in 4 (4.3%), and abdominal surgery in 35 (37.2%). Approval for this study was obtained from the Institutional Review Board of National Taiwan University Hospital, Taipei, Taiwan (no. 31MD03).
Clinical assessments
Disease severity was assessed using the Sequential Organ Failure Assessment (SOFA) score [11]. Day 0 (D0) was defined as the day after the last session of acute dialysis. Day 1 was the day of the last session. The SOFA score and renal function parameters [i. e., amount of urine, blood urea nitrogen (BUN), and serum creatinine (sCr)] were assessed on the day of intensive care unit admission, on the day of initial postoperative dialysis, and on D0. Older age was defined as that over 65 years [12, 13], chronic kidney disease (CKD) as sCr of 1.5 mg/dl or greater before hospital admission [14], oliguria as a urine amount less than 100 ml in 8 h [15] on D1. A daily diet of 1.0–1.2 g protein/kg was prescribed for patients. To estimate the response to diuretics we calculated the total daily dose of loop diuretic (in furosemide equivalents) divided by the total urine output in milliliters (index of diuretic responsiveness). For the calculation of diuretics and urine output 1 mg bumetanide was considered to be equivalent to 40 mg furosemide [16].
Organ failure was classified according to the following findings: respiratory failure, a partial pressure of arterial blood gas oxygen/fraction of inspired oxygen ratio (PaO2/FIO2) higher than 200; coagulopathy, platelets less than 50 × 103/mm3, hepatic failure, total bilirubin above 6.0 mg/dl; central nervous system dysfunction; Glasgow Coma Score greater than 9 [17]; cardiac failure, low cardiac output with a central venous pressure more than 12 mmHg, and a dopamine equivalent greater than 5 μg/kg per minute [18]. Sepsis was defined as persistence or progression of the signs and symptoms of the systemic inflammatory response syndrome with a documented or presumed persistence of infection [19].
Early redialysis, defined as relapsed need for RRT within 30 days after D0, was considered the primary outcome variable. Hospital mortality and hospital redialysis were considered as the secondary outcomes.
Interventions of acute RRT
The indications for dialysis were: (a) azotemia (BUN > 80 mg/dl and sCre > 2 mg/dl) with uremic symptoms (n = 40), (b) fluid overload with a central venous pressure level higher than 12 mmHg or pulmonary edema with a PaO2/FIO2 greater than 300 (n = 32), (c) hyperkalemia (serum K+ > 5.5 mmol/l) despite medical treatment (n = 9), (d) oliguria (urine < 100 ml/8 h) with or without use of diuretics (n = 31), and (e) acidosis (pH < 7.2 in arterial blood gas; n = 4). There were no generally accepted criteria for terminating RRT in ARF. In our groups to fulfill the following criteria was considered mandatory criteria for patients to be weaned off dialysis: (a) serum K+ greater than 5.5 mmol/l, (b) arterial blood gas pH higher than 7.33 or \( {\text{HCO}}_{3^{-}} \) above 23 mEq/l, (c) sCr less than 5 mg/dl, (d) a trend of decreasing sCr, and (e) urine output more than 100cc/8 h without the use of diuretics or more than 150 cc/8 h with diuretics and a trend of increasing urine output on the dialysis day with an evaluation by the attending physician. The diuretics group was defined as patients who received more than 20 mg loop diuretics (furosemide) daily at least 2 days before cessation of dialysis.
The dialysis modality was chosen according to the hemodynamics of patients. Continuous venovenous hemofiltration (CVVH) was used if the dose of inotropic equivalent [20, 21] of more than 15 points was required to maintain systolic blood pressure up to 120 mmHg. CVVH was performed with high-flux filters (Hemofilter, PAN-10, Asahi Kasei, Japan) using HF 400 (Infomed, Geneva, Switzerland) and a hemofiltration flow of 35 ml/kg per hour with a blood flow of 200 ml per minute. Replacement fluid was bicarbonate-buffered and was administered predilutionally at a dynamically adjusted rate to achieve the desired fluid therapy goals. Default composition was 142 mEq/l Na, 33 mEq/l bicarbonate, 1.4 mEq/l Mg, and 2.6 mEq/l Ca. Hemodialysis was performed using low-flux polysulfone hemofilters (KF-18C, Kawasumi Laboratories, Japan). Conventional intermittent hemodialysis was performed for 4 h except for the first and second sessions with a blood flow of 200 ml/min and a dialysate flow of 500 ml/min [21]. Hemodialysis adequacy assessment was measured: KT/V = [(in vitro urea clearance) × (prescribed time)]/predialysis total body water [21, 22]. The urea distribution volume is roughly equal to the total body water. Vascular access was obtained by percutaneous placement of a double lumen catheter.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation. The unpaired Student's t-test was used to analyze continuous data, and Fisher's exact test was used to analyze categorical data. Statistical analyses were performed with SPSS for Windows version 12.0 (SPSS, Chicago, Ill., USA). Significant risk factors determined by univariate analysis were included in the multivariate analysis by applying a multiple logistic regression analysis with a stepwise forward method to obtain the variables that were independently associated with the failed weaning. Calibration of the model was assessed by Cg, a goodness-of-fit statistic test described by Hosmer–Lemeshow [23], and discrimination capability was evaluated by determination of the area under the receiver operating characteristic curve [24]. two-way analysis of variance with repeated measures over time was used to compare the changes between successful and failed weaning groups. The Kaplan–Meier (product-limit) method was used to estimate freedom from redialysis. A p-value less than 0.05 was considered statistically significant.
Results
Patient characteristics
The ratio of diuretic use at the time of dialysis cessation was also the same between groups. However, failed weaning patients were older ( p = 0.004), had a longer duration of dialysis ( p = 0.007), higher SOFA score on D0 ( p < 0.001), higher BUN on D0 ( p = 0.013), and less urine output on D1 (598 ± 700 vs. 1435 ± 1172 ml/d, p < 0.001). They also had a higher rate of respiratory (40.0% vs. 17.2%, p = 0.018) and cardiac failure (50.0% vs. 28.1%, p = 0.034) at the start of dialysis than patients successfully weaned (Table 1, 2). In the redialysis group (n = 30) the indications for resumption of dialysis were azotemia (n = 13), oliguria (n = 11), fluid overload (n = 4), and electrolyte imbalance (n = 2). Eighteen (60%) patients resumed dialysis with inotropic agents. The mean duration from weaning to redialysis was 10.1 ± 6.1 days. Among these patients eight (26.7%) had sepsis before redialysis. Neither nephrotoxic agents nor radiocontrast materials were used after the initial cessation of dialysis, with the exception of five patients (16.7%) who were administered vancomycin for the treatment of sepsis.
Factors related to weaning failure
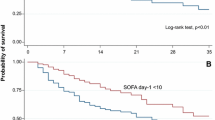
The independent predictors for resuming dialysis were as follows: longer duration of dialysis ( p = 0.005), higher SOFA score on D0 ( p = 0.003), oliguria (urine < 100cc/8 h, p = 0.039) on D1, and age over 65 years ( p = 0.008; Table 3). The multivariate logistic regression equation was: log odds of failed weaning from acute dialysis = 1.848 × older age + 0.361 × SOFA score on D0 + 1.429 × oliguria on D1 + 0.056 ± dialysis duration + 4.188. This model had a good calibration, as estimated by the Hosmer–Lemeshow goodness-of-fit (Cg = 2.657, p = 0.954), and good discriminative power (area under the receiver operating characteristic curve 0.880 ± 0.036, p < 0.0001; Fig. 1).
The two-way analysis of variance with repeated measurements over time revealed significantly less decline in SOFA scores (group × time interaction, p = 0.006) and less increase in urine output (group × time interaction, p = 0.001) but no significant difference in BUN (between groups, p = 0.096) in the patients who failed weaning. There were no differences in diuretic use (25.8% vs. 41.3%, p = 0.174) and the index of diuretic responsiveness on D1 (0.51 ± 2.0, vs. 0.65 ± 0.12, p = 0.088) between oliguria and nonoliguric groups. Kaplan–Meier analysis showed a significant difference in returning to dialysis between patients with or without oliguria on D1 (log rank, p < 0.001; Fig. 2).
Outcome
In the redialysis group 15 patients died in hospital while on dialysis (41.1 ± 39.2 days), 12 were discharged from the hospital without dialysis (with a mean redialysis period of 20.5 ± 12.3 days), and 3 died during the same admission without dialysis. In 64 patients who were successfully weaned from dialysis for more than 30 days 13 died during the same hospital stay (60.8 ± 29.5 days from D0). Fifty-one patients survived to discharge; however, three of these patients resumed dialysis before discharge from the hospital. Successfully weaned patients had a higher rate of survival to hospital discharge than did the patients who failed weaning (79.7% vs. 40.0%, p < 0.001; Table 3).
Discussion
The incidence of ARF after surgery ranges from 5% to 30%, and ARF in surgical patients is associated with a mortality rate of 60–90% [25, 26]. Moreover, the survivors of postoperative ARF may develop endstage renal disease [27]. While recovery to independence from RRT occurs in approx. 38–87% at discharge from ICU [1], there have been few epidemiological studies that have specifically investigated factors predictive of renal recovery. In our study of postoperative acute dialysis more than one-third of patients resumed dialysis within 1 month after weaning for longer than 5 days.
However, the factors that underlie successful weaning from acute dialysis have not yet been defined. The reason that such factors have been elusive may be because ARF patients are often labeled as dialysis-dependent and rarely regain their renal function [28]. We identified four factors that were independently associated with dialysis weaning failure. The assessment of renal function remains rudimentary as there is no method available at present to monitor real-time glomerular filtration rate. The only practical measurement is urine output [4]. Urine output is not only a simple and early prognostic index for ARF patients requiring dialysis [7, 17, 29, 30], but oliguria is also a marker for the severity of renal and multiorgan injury [8]. Although the perioperative urine output may not be predictive of postoperative renal function [31], the patients with successful weaning from acute RRT in the current study showed a significant higher urine output on the day of dialysis cessation. Those patients successfully weaned regained normal urine output more quickly, as was also reported in a retrospective cohort study of chronic kidney disease patients who required acute hemodialysis [32]. Diuretics increase urine output; however, our results showed that the effect of furosemide on a patient's renal recovery at the time dialysis was withdrawn was equivocal [16].
Although dialysis is the mainstay of supportive care in patients with severe ARF, performance of this life-sustaining treatment can have untoward effects that contribute to the prolongation of renal failure or impede the ultimate recovery of renal function [33]. Renal biopsy in patients with prolonged ARF managed by using hemodialysis demonstrates regions of fresh tubular necrosis days-to-weeks after the initial inciting insult [34]. The association of shorter dialysis duration with improved outcomes is likely a reflection of early renal recovery and/or improved hemodynamic stability, which facilitate the successful cessation of dialysis [8].
Age and disease severity scores have been reported as risk factors for poor prognosis in ARF [7, 17, 35]. Older patients with increasing comorbidities have been proposed as being causal [2]. Although failure of a single organ does not significantly contribute to the failed cessation of RRT, a number of dysfunctional organs do, as based on SOFA scores, thereby stressing the role of associated organ failure as an important prognostic determinant in postoperative patients with regard to both mortality and morbidity [6, 17, 30].
In the current study BUN was not an independent risk factor for resumption of dialysis; however, patients who failed weaning from dialysis had higher BUN levels than patients in the successfully weaned group on D0. A higher BUN may be associated with increased protein catabolism, a subtle sign of metabolic stress (e.g., gastrointestinal bleeding, nutritional supplementation, and corticosteroid use) [7]. Despite the greater hemodynamic stability in continuous RRT-treated patients, dialysis modality does not determine the success of weaning from postoperative acute dialysis, although a trend toward complete renal recovery is observed with continuous therapy [35–37].
The main limitation in a study such as the current one is in part due to the great heterogeneity of the patient population, in particular, the causes of ARF, the severity of kidney injuries, and the accompanying chronic comorbid factors [2]. Therefore we defined early redialysis as the primary endpoint because other than the underlying patient's characteristics, the need for resumption of dialysis is always influenced by subsequent events that can result in new insults to the kidney with a long hospital stay, especially in intensive care units. Nevertheless, there were several other important limitations to this study. First, we were limited by the frequency of measurement of some physiological and laboratory variables that may be associated with redialysis. Second, data on urine output were not appropriate. Although we recorded urine output on the day of the last dialysis, dialysis modality and ultrafiltration influences the volume status and urine output and therefore likely reduced the predictive power of our model. Third, the weaning from dialysis was not based on standardized criteria and that the experience comes from a single center, although general rules were followed. Further prospective studies should be carried out to reconcile weaning early (reducing the period of dialysis) or weaning late (permitting the urine output to increase).
In conclusion, albeit retrospective, our study is the first to address a clinically relevant question of risk factors for redialysis after initially weaning form postoperative acute RRT. Our data indicate that surgical patients with ARF may remain ill with an increased risk for resuming dialysis after temporarily being taken off acute RRT. Older age, higher SOFA scores, oliguria, and a longer dialysis-dependent period were independent predicators of early redialysis in postoperative ARF patients.
References
Bagshaw SM, Mortis G, Godinez-Luna T, Doig CJ, Laupland KB (2006) Renal recovery after severe acute renal failure. Int J Artif Organs 29:1023–1030
Schiffl H (2006) Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant 21:1248–1252
Bagshaw SM (2006) Epidemiology of renal recovery after acute renal failure. Curr Opin Crit Care 12:544–550
Carmichael P, Carmichael AR (2003) Acute renal failure in the surgical setting. ANZ J Surg 73:144–153
Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM (1995) Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155:1505–1511
Cosentino F, Chaff C, Piedmonte M (1994) Risk factors influencing survival in ICU acute renal failure. Nephrol Dial Transplant [Suppl] 9(4):179–182
Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM (2002) Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13:1350–1357
Wald R, Deshpande R, Bell CM, Bargman JM (2006) Survival to discharge among patients treated with continuous renal replacement therapy. Hemodial Int 10:82–87
Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM (2005) Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 353:349–361
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L (1999) Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100:1043–1049
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710
Wu VC, Huang JW, Hsueh PR, Yang YF, Tsai HB, Kan WC, Chang HW, Wu KD (2005) Renal hypouricemia is an ominous sign in patients with severe acute respiratory syndrome. Am J Kidney Dis 45:88–95
Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T (2005) Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care 9:R700–R709
Lin YH, Pao KY, Wu VC, Lin YL, Chien YF, Hung CS, Chen YJ, Liu CP, Tsai IJ, Gau CS, Wu KD, Hwang JJ (2007) The influence of estimated creatinine clearance on plasma homocysteine in hypertensive patients with normal serum creatinine. Clin Biochem 40:230–234
Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ (2004) Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur J Cardiothorac Surg 26:1027–1031
Mehta RL, Pascual MT, Soroko S, Chertow GM (2002) Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 288:2547–2553
de Mendonca A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F (2000) Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26:915–921
Chen YS, Ko WJ, Lin FY, Huang SC, Chou TF, Chou NK, Hsu RB, Wang SS, Chu SH (2001) Preliminary result of an algorithm to select proper ventricular assist devices for high-risk patients with extracorporeal membrane oxygenation support. J Heart Lung Transplant 20:850–857
Wu VC, Wang YT, Wang CY, Tsai IJ, Wu KD, Hwang JJ, Hsueh PR (2006) High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Clin Infect Dis 42:66–72
Bone RC (1996) Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med 125:680–687
Wu VC, Ko WJ, Chang HW, Chen YS, Chen YW, Chen YM, Hu FC, Lin YH, Tsai BR, Wu KD (2007) Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: effect on postoperative outcomes. J Am Coll Surg 205(2):266–276
Himmelfarb J, Evanson J, Hakim RM, Freedman S, Shyr Y, Ikizler TA (2002) Urea volume of distribution exceeds total body water in patients with acute renal failure. Kidney Int 61:317–323
Lemeshow S, Hosmer DW Jr (1982) A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol 115:92–106
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M (2004) Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 15:1597–1605
Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT (1998) Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128:194–203
Leacche M, Rawn JD, Mihaljevic T, Lin J, Karavas AN, Paul S, Byrne JG (2004) Outcomes in patients with normal serum creatinine and with artificial renal support for acute renal failure developing after coronary artery bypass grafting. Am J Cardiol 93:353–356
Agraharkar M, Nair V, Patlovany M (2003) Recovery of renal function in dialysis patients. BMC Nephrol 4:9
Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ (1996) Acute renal failure in intensive care units-causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med 24:192–198
Liano F, Pascual J (1996) Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50:811–818
Alpert RA, Roizen MF, Hamilton WK, Stoney RJ, Ehrenfeld WK, Poler SM, Wylie EJ (1984) Intraoperative urinary output does not predict postoperative renal function in patients undergoing abdominal aortic revascularization. Surgery 95:707–711
Chen JT, Wu MS, Chen YM, Tsai TJ (2004) Analysis of renal outcome in patients with acute on chronic renal failure requiring emergent hemodialysis (abstract). J Intern Med Taiwan 15:115–124
Palevsky PM, Baldwin I, Davenport A, Goldstein S, Paganini E (2005) Renal replacement therapy and the kidney: minimizing the impact of renal replacement therapy on recovery of acute renal failure. Curr Opin Crit Care 11:548–554
Solez K, Morel-Maroger L, Sraer JD (1979) The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 58:362–376
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, Kaplan RM (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60:1154–1163
Bell M, Granath F, Schon S, Ekbom A, Martling CR (2007) Continuous renal replacement therapy is associated with less chronic renal failure than intermittent haemodialysis after acute renal failure. Intensive Care Med 33:773–780
Uchino S, Bellomo R, Kellum JA, Morimatsu H, Morgera S, Schetz MR, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-Van Straaten HM, Ronco C (2007) Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs 30:281–292
Acknowledgements
The National Taiwan University Surgical ICU Acute Renal Failure Study Group includes Vin-Cent Wu, MD, Wen-Je Ko, MD, PhD, Yen-Hung Lin, MD, Chih-chung Shiao, MD, Yu-Feng Lin, MD, Yung-Wei Chen, MD, Yih-Sharng Chen, Yung-Ming Chen, MD, Pi-Ru Tsai, Miss, Hung-Bin Tsai, MD, Tzong-Yann Lee, MD, Jann-Yuan, Wang, MD, Fu-Chang Hu, M.S., Sc.D. and Kwan-Dun Wu, MD, PhD.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The complete list of NSARF members is provided in the Acknowledgements.
This study was financially supported by the Improving Dialysis Quality Research Funds, the Ta-Tung Kidney Foundation and Taiwan National Science Council (grant: NSC 95-2314-B-002-166). There are no conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, VC., Ko, WJ., Chang, HW. et al. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med 34, 101–108 (2008). https://doi.org/10.1007/s00134-007-0813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0813-x