Abstract
Objectives
To identify the proportion of critically ill patients able to consent to participation in a randomised controlled trial (RCT) and to assess to what extent patient consent and relative assent processes could be conducted according to ethics committee permissions.
Design
Descriptive study nested in an RCT.
Setting
Fifty-six UK intensive care units participating in the PAC-Man trial.
Patients and participants
First 500 patients consecutively enrolled into PAC-Man.
Measurement and results
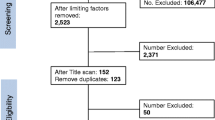
The outcome measures were patient consent and/or relative assent. Of the 498 patients included, 13 (2.6%) provided consent before randomisation. Of the remaining 485 patients, relative assent was obtained for 394 patients (81.2%), and refused post-randomisation for 3 patients (0.6%). No relatives were available for 15 patients (3.1%), and it was unclear from documentation whether relative assent had been obtained for 73 patients (15.1%). Of the 482 patients who did not provide consent prior to randomisation, 188 (39%) survived. Of these, 175 (93.1%) gave retrospective informed consent, six (3.2%) refused, and seven (3.7%) did not regain mental competency.
Conclusions
A very small proportion of patients were able to give consent before randomisation. Due to the high in-hospital mortality (60.6%), only around one third of the remaining patients could provide consent retrospectively. This study demonstrates difficulties experienced in obtaining consent from critically ill patients to participate in medical research and raises important issues about the ethical basis of the consent process in critical care.

Similar content being viewed by others
References
Freedman B (1987) Equipoise and the ethics of clinical research. N Engl J Med 317:141–145
Beauchamp TL, Childress JF (1994) Principles of biomedical ethics. 4th edn. Oxford University Press, New York
Silverman HJ, Luce JM, Schwartz J (2004) Protecting subjects with decisional impairment in research: the need for a multifaceted approach. Am J Respir Crit Care Med 169:10–14
Annane D, Outin H, Fisch C, Bellissant E (2004) The effect of waiving consent on enrollment in a sepsis trial. Intensive Care Med 30:321–324
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K, PAC-Man study collaboration (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet 366:472–477
Baren JM, Anicetti JP, Ledesma S, Biros MH, Mahabee-Gittens M, Lewis RJ (1999) An approach to community consultation prior to initiating an emergency research study incorporating a waiver of informed consent. Acad Emerg Med 6:1210–1215
Lewis RJ, Berry DA, Cryer H, Fost N, Krome R, Washington GR, Houghton J, Blue JW, Bechhofer R, Cook T, Fisher M (2001) Monitoring a clinical trial conducted under the Food and Drug Administration regulations allowing a waiver of prospective informed consent: the Diaspirin Cross-Linked Hemoglobin Traumatic Hemorrhagic Shock Efficacy Trial. Ann Emerg Med 38:397–404
Booth MG, Lind A, Read E, Kinsella J (2005) Public perception of emergency research: a questionnaire. Eur J Anaesthesiol 22:933–937
Smithline HA, Gerstle ML (1998) Waiver of informed consent: a survey of emergency medicine patients. Am J Emerg Med 16:90–91
McClure KB, DeIorio NM, Gunnels MD, Ochsner MJ, Biros MH, Schmidt TA (2003) Attitudes of emergency department patients and visitors regarding emergency exception from informed consent in resuscitation research, community consultation, and public notification. Acad Emerg Med 10:352–359
Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N, Mazairac G, Laloe V, Munoz-Sanchez A, Arango M, Hartzenberg B, Khamis H, Yutthakasemsunt S, Komolafe E, Olldashi F, Yadav Y, Murillo-Cabezas F, Shakur H, Edwards P, CRASH trial collaborators (2004) Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 364:1321–1328
CESAR Trial, http://www.cesar-trial.org (accessed 25 October 2005)
TracMan Trial,http://www.tracman.org.uk (accessed 25 October 2005)
CRASH Trial Management Group (2004) Research in emergency situations: with or without relatives' consent. Emerg Med J 21:703
Mariner WK (1995) Research in emergency care without consent: new proposed FDA rules. Lancet 346:1505–1506
Chalmers I, Silverman WA (1987) Professional and public double standards on clinical experimentation. Control Clin Trials 8:388–391
Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001, http://eudract.emea.eu.int/docs/Dir2001-20_en.pdf (accessed 25 October 2005)
Medicines for Human Use (Clinical Trials Regulations) 2004. Informed consent in clinical trials (7th February 2005), http://www.corec.org.uk/applicants/help/docs/Informed_Consent_in_CTIMPsv1.1.doc (accessed 25 October 2005)
Corrigan OP, Williams-Jones B (2003) Consent is not enough – putting incompetent patients first in clinical trials. Lancet 361:2096–2097
Chalmers I (2003) Provision of consent. Lancet 362:663–664
Klepstad P, Dale O (2006) Further restrictions for ICU research. Intensive Care Med 32:175
Druml C (2004) Informed consent of incapable (ICU) patients in Europe: existing laws and EU Directive. Curr Opin Crit Care 10:570–73
Lemaire F (2004) A waiver of consent for intensive care research. Intensive Care Med 30:177–79
Coats TJ, Shakur H (2005) Consent in emergency research: new regulations. Emerg Med J 22:683–685
Foex BA (2001) The problem of informed consent in emergency medicine research. Emerg Med J 18:198–204
Acknowledgements
We wish to thank the patients and staff at participating hospitals and the PAC-Man Study Steering Committee, and David Harrison for extra analyses. We also wish to thank Lesley Morgan (Trial Manager for the TracMan Trial) and Ian Roberts (Principal Investigator for the CRASH Trial) for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding: The PAC-Man trial (HTA Project No. 97/08/03) was funded by the UK National Health Service Research & Development Health Technology Assessment Programme. (Disclaimer: The views and opinions expressed in this article do not necessarily reflect those of the National Co-ordinating Centre for Health Technology Assessment.) The NHS Research & Development Health Technology Assessment Programme had no involvement in the design, conduct and analysis of the study.
Contributors: S.E. Harvey conceived and designed the study and participated in the analysis and interpretation of data and in writing the paper. D. Elbourne participated in the analysis and interpretation of data and in writing the paper. J. Ashcroft and C.M. Jones collected the data and participated in the interpretation of data and in writing the paper. K. Rowan conceived and designed the study and participated in the interpretation of data and in writing the paper. S.E. Harvey is the guarantor of the paper and accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish.
Competing interest statement: All authors declare no conflict of interest.
Ethics approval: The PAC-Man trial (Application reference number MREC/00/2/83) was approved by the North Thames Multi-centre Research Ethics Committee on 12 March 2001, as well as all relevant Local Research Ethics Committees, and Research & Development Departments at collaborating hospitals.
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-006-0359-3
The authors wrote this article on behalf of the PAC-Man Study Collaboration
Electronic supplementary material
134_2006_358_MOESM1_ESM.doc
Electronic Supplementary Material is available in the online version of this article at http://dx.doi.org/10.1007/s00134-006-0358-4 and is accessible for authorized users.
Rights and permissions
About this article
Cite this article
Harvey, S.E., Elbourne, D., Ashcroft, J. et al. Informed consent in clinical trials in critical care: experience from the PAC-Man Study. Intensive Care Med 32, 2020–2025 (2006). https://doi.org/10.1007/s00134-006-0358-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0358-4