Abstract
Objective
In acute respiratory distress syndrome the body posture effects on pressure-volume (PV) curves are still unclear. We examined the effects of prone position on inflation PV curves and their potential relationships with postural alterations in gas exchange.
Design and setting
Prospective study with patients serving as their own controls in a university-affiliated 30-bed intensive care unit.
Patients and participants
Thirteen anesthetized, paralyzed, semirecumbent, mechanically ventilated patients with early/severe/diffuse ARDS.
Interventions
Sequential body posture changes: preprone semirecumbent, prone, and postprone semirecumbent.
Measurements and results
In each posture hemodynamics, gas exchange, and lung volumes were determined before/during removal and after restoration of positive end-expiratory pressure (PEEP=10.2±0.6 cmH2O). At zero PEEP PV curves of respiratory system, lung, and chest wall were constructed. Prone position vs. preprone semirecumbent resulted in significantly reduced pressure at lower inflection point of lung PV curve (2.2±0.2 vs. 3.7±0.5 cmH2O) and increased volume at upper inflection point (0.87±0.03 vs. 0.69±0.05 l). Postural reduction in lower inflection point pressure of lung PV curve was the sole independent predictor of pronation-induced increases in PaO2/FIO2 (R2=0.76). PaO2/FIO2 increases were also significantly related with increases in functional residual capacity (R2=0.60).
Conclusions
In early/severe/diffuse ARDS prone position reduces lower inflection point pressure and increases upper inflection point UIP volume of the lung PV curve. Lower inflection point pressure reductions explain oxygenation improvements, which are also associated with a postural increase in functional residual capacity.
Similar content being viewed by others
Introduction
In acute respiratory distress syndrome (ARDS) the analysis of the quasistatic pressure-volume (PV) curve may aid in ventilatory settings adjustment [1, 2, 3, 4, 5, 6, 7]. Inflation PV curve-based setting of positive end-expiratory pressure (PEEP) has been shown in lavage-injured sheep to be most effective in maintaining gas exchange and minimizing lung injury [8]. In ARDS the prone position results in decreased plateau transpulmonary pressure, increased static lung compliance, and improved gas exchange [9, 10, 11, 12, 13]. Previously demonstrated pronation benefits on lung mechanical behavior suggest that prone position improves the shape of lung inflation PV curves. However, to date relevant data are scarce and conflicting [14].
We theorized that in severe ARDS, prone position would result in (a) leftward displacement of the lung inflation PV curve and (b) decreased pressure at the lower inflection point (LIP) and increased volume at the upper inflection point (UIP) of the lung PV curve. We also sought to determine any relationship between the postural PV curve changes and any concomitant alterations in gas exchange.
Materials and methods
Patients
Institutional approval and informed, written next-of-kin consent were obtained. The study enrolled 13 consecutive, orotracheally intubated (cuffed endotracheal tube no. 7.5–8.5) and mechanically ventilated (Siemens 300C, Siemens, Berlin, Germany) patients with early-onset, early-stage (i.e., within 7 days of onset), and severe ARDS (Table 1) [9, 15, 16, 17]. Inclusion criteria were a PaO2/FIO2 ratio of100 mmHg or less, “white lungs” feature on chest radiography, and/or diffuse attenuations on thoracic computed tomography [16]. Exclusion criteria were age under 18 years, pregnancy, intracranial hypertension, burns over more than 30% of body surface area, spine fractures, smoking and/or chronic respiratory disease, hemodynamic instability (systolic blood pressure <95 mmHg), chest wall abnormalities and pneumothorax, chronic liver disease (Child-Pugh class C), neuromuscular disease impairing spontaneous breathing, sickle cell disease, and body mass index greater than 27.5.
Electrocardiographic lead II, intra-arterial/pulmonary artery pressures, cardiac index (744HF75 CCOmbo Swan-Ganz catheter, Edwards Lifesciences, Irvine, Calif., USA), and peripheral oxygen saturation (SaO2) were monitored continuously. Esophageal and gastric balloons were inserted and their correct placement as previously verified [18, 19, 20]. Additional information is provided in the Electronic Supplemental Material.
Protocol
On enrollment the patients were anesthetized/paralyzed [9] and ventilated in a baseline preprone semirecumbent position (45° inclination). Baseline ventilation tidal volume (Table 1) was titrated to a plateau airway pressure less than 30 cmH2O or kept within 6–6.5 ml/kg if plateau pressure exceeded 30 cmH2O. Inspiratory flow ranged within 0.75–0.90 l/s. Respiratory rate (range 20–30/min) was adjusted to maintain pHa at or above 7.30. PEEP was set at 2 cmH2O above a mean LIP determined graphically [2, 7] by two independent clinicians within 24 h before enrollment. These physicians constructed respiratory system PV curves at zero PEEP with multiple inspiratory airway occlusion [21]; administered inflation volume ranged within 0.1–0.8 l, because the main objective was to determine the LIP.
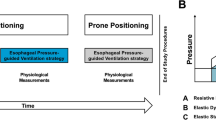
Patients were sequentially studied in three body postures: (a) preprone semirecumbent, (b) prone, and (c) postrpone semirecumbent (45° inclination). For each posture the study duration was 135–140 min. Measurements were performed during PEEP application, zero PEEP, and after original PEEP restoration. Minimal allowable SaO2 was 89%. Following pronation a roll and a pillow were placed under the upper chest wall and pelvis, respectively [9, 10]. Figure 1 illustrates the time sequence of investigational interventions. The time points of recruitment with inflation volumes of 1.0 l or greater (see below) [9, 12, 22], and the periods of volume history standardization are also indicated. Four minutes prior to PEEP removal, inspired oxygen fraction (FIO2) was increased to 1.0. FIO2 was restored to its original level (Table 1) within 5–15 min after PEEP restoration (Fig. 1). Following PEEP removal volume history was standardized over only 5 min, because: (a) in ARDS, a single, prolonged expiration to zero PEEP leads to reversal of PEEP-induced recruitment [6], implying rapid respiratory system equilibration to zero PEEP conditions; (b) the expiration just before PV curve construction was prolonged [6] (see below); and (c) by protocol, the time of uninterrupted zero PEEP ventilation should be minimized. If hemodynamics/gas exchange determinations (required time=6 min) were performed immediately after the 5-min period of equilibration at zero PEEP, the time of uninterrupted zero PEEP ventilation would have risen to 11 min (Fig. 1).
Time sequence of investigational interventions for each body posture. Lung volumes included determination of (a) physiological dead space (2 min of collection of expired gases into a Douglas bag and determination of CO2 concentration with an infrared analyzer); (b) functional residual capacity (FRC) change (passive exhalation from end-expiratory lung volume to FRC lasting 10 s or less; the reaching of FRC level was confirmed by an airway pressure of 0 cmH2O during an expiratory occlusion of 2 s); and (c) FRC by helium-dilution (corresponding to approx. 2 min of ventilation with high inflation volumes of 1.0–1.3 l). In all postures during external positive end-expiratory pressure (PEEPe) application inspired oxygen fraction (FIO2) was identical to that reported in Table 1 (baseline FIO2); during the last 4 min of the first PEEPe period and during the first 15 min of the zero PEEPe period, ventilator FIO2 was increased to 1.0; during the last 2 min of the zero PEEPe period (and during all FRC measurements), FIO2 was 0.87, because FRC was measured with the use of 13% helium in oxygen [9, 10]; following PEEPe restoration, ventilator FIO2 was readjusted to baseline FIO2 within 5–15 min in all cases, with peripheral oxygen saturation being always maintained at or above 89%. Periods of volume history standardization (VHS) and time points of administration of recruitment maneuver(s) (RM) are highlighted (see also text)
Complete sets of measurements were obtained from all patients and no protocol-related complications [11, 23] occurred. Eight patients were weaned from mechanical ventilation 15.8±1.2 days after its institution and discharged from intensive care unit after another 2.5±0.2 days. Five patients died of multiple system organ failure (Table 1).
PV curves
Flow, volume, and airway, esophageal, and gastric pressures were measured as previously described [9, 24]. Esophageal pressure measurements’ reliability was as previously tested [9, 24]. In each posture inflation PV curves of the respiratory system, lung, and chest wall were constructed at zero PEEP (Fig. 1). Functional residual capacity (FRC) was reached during a prolonged (5–10 s) exhalation [25]; this was confirmed by an expiratory occlusion pressure of 0 cmH2O [25]. A 2-l calibration syringe filled with 100% oxygen was then connected to the endotracheal tube. PV curves were obtained by sequentially adding 50–100 ml in steps of 2–3 s until the lungs were inflated with 1.2 l. Procedure duration was 40–45 s. Maximal allowable airway and transpulmonary pressures were 50 and 35 cmH2O, respectively.
PV curve data analysis
PV diagrams for the respiratory system, lung, and chest wall were constructed. Regression lines for three or four consecutive points were determined with the Statistical Package for Social Sciences version 10.0 (SPSS, Chicago, Ill., USA). The slopes of the regression lines were determined in Adobe Photoshop 5.0 (Adobe Systems, San Jose, Calif., USA) with the Measure Tool. On confirmation of a consistent change in regression line slope of 20% or more [19] the intersection point of the linear tracts identifying the zones of initial and maximal compliance was identified as LIP. Accordingly, the UIP was identified at the intersection of the linear tracts identifying the zones of final and maximal compliance. Linear tracts were drawn as regression line extensions with the Line Tool. Following LIP and UIP determination the identity of the slopes of the regression lines and their extensions was confirmed with the Measure Tool. Initial (starting) static compliance was determined as inflation volume at LIP divided by pressure at LIP. For the respiratory system and lung, maximal static compliance was determined as inflation volume difference between UIP and LIP divided by the corresponding pressure difference; final static compliance was determined as inflation volume difference between the PV curve’s maximal pressure point and UIP divided by the corresponding pressure difference. Chest wall PV curves did not exhibit UIPs. Therefore maximal static chest wall compliance was determined as inflation volume difference between the PV curve’s maximal pressure point and LIP divided by the corresponding pressure difference.
Each diagram was analyzed by four independent observers. Computer files containing diagrams were named (encoded) according to patient initials, body posture, and respiratory system component. Following completion of diagram evaluations, diagram codes were broken, and means of observer-determined variable values were analyzed statistically.
Hemodynamics, gas exchange, and lung volumes
Measurements were performed over 6-min periods (Fig. 1). Hemodynamic measurements included arterial, central venous, and pulmonary artery pressures, cardiac output/index, and mixed-venous oxygen saturation (SvO2). Arterial and mixed-venous blood gas analysis (ABL System 625, Radiometer, Copenhagen, Denmark) was concomitantly performed. Physiological dead space, FRC change, and FRC were measured as previously described [9, 10, 12, 24, 26].
Statistics
Data sets from different body postures or end-expiratory pressure were compared using SPSS by repeated-measures analysis of variance for two within-group factors, followed by Scheffé’s test as appropriate. Significance was set at p<0.05. Multiple linear regression analysis was performed by stepwise method; variable entry and removal criteria were p<0.05 and p<0.1, respectively. Data are reported as mean ±standard error.
Results
As previously [9], gastric and esophageal pressures at FRC, and mean maximal amplitude and mean rise rate in “cardiac oscillations” of esophageal pressure tracings [9, 24] did not differ significantly between study postures (Table 2). Consequently, (a) abdominal movement restriction was effectively minimized in the prone position [9, 10, 24], and (b) the initial, correct esophageal balloon positioning relative to the heart was maintained throughout the study period, and measurements of esophageal pressure changes were as accurate as possible in all postures [9, 24]. The esophageal balloon technique has previously been adopted by us and others and shown to be adequate also in the prone position [9, 10, 27, 28, 29].
Reproducibility of computerized methodology
Measurements of the LIP pressures of the respiratory system, lung, and chest wall exhibited average interobserver variabilities of 2.2–2.7%, 4.1–9.6%, and 3.7–4.3%, respectively. For the respiratory system and lung average interobserver variability values of UIP pressure measurements ranged within 1.1–1.3% and 1.3–2.2%, respectively. Thus LIP and UIP pressures were consistently determined within 0.37 and 0.40 cmH2O, respectively. (See also ESM.)
PV curves
Figure 2 displays average PV curves. Prone position resulted in leftward shifting of the respiratory system and lung PV curves. Table 3 summarizes results on determined variables of the respiratory system, lung, and chest wall PV curves. Main results were as follows: (a) Respiratory system PV curves: relative to preprone/postprone semirecumbent, the UIP corresponded to similar pressure but significantly higher volume in the prone position. Final respiratory system compliance was higher in prone vs. preprone semirecumbent. (b) Lung PV curves: the LIP corresponded to lower pressure/volume in prone vs. preprone/postprone semirecumbent (Fig. 3). The UIP corresponded to similar pressure but higher volume in prone than in preprone/postprone semirecumbent. Starting and maximal lung compliance were higher in prone than in preprone/postprone semirecumbent. Final lung compliance was higher in prone vs. preprone semirecumbent. (c) Chest wall PV curves: maximal chest wall compliance was lower in prone than in preprone semirecumbent. The relative contribution of the LIP of the chest wall PV curve to the respiratory system LIP (Fig. 3) was higher in prone than in preprone/postprone semirecumbent (69.3±2.0% vs. 59.6±1.4%/52.4±1.5%, respectively, p<0.001 for both).
Hemodynamics, gas exchange, and lung volumes
Hemodynamic variables were unaffected by posture change (data not shown). PEEP caused significant drops in mean arterial pressure (by 8–12%) and rises in pulmonary artery pressure and pulmonary vascular resistance index (by 14–19% and 14–44%, respectively). Prior to PEEP removal PaO2/FIO2, PaCO2, shunt fraction, physiological dead space, and FRC were improved in prone and postprone semirecumbent relative to preprone semirecumbent. Following PEEP removal and after PEEP restoration, gas exchange, and lung volumes were improved in prone relative to preprone and postprone semirecumbent (Table 4). With respect to posture change, the results on end-expiratory lung volume (EELV) were similar to those on FRC (Table 4).
The principal consequences of PEEP removal were (a) significant reductions in PaO2/FIO2 only in the semirecumbent postures, (b) significant increase in shunt fraction in all postures, and (c) significant FRC reduction only in postprone semirecumbent (Table 4). In postprone semirecumbent oxygenation and shunt fraction exhibited the maximal observed deterioration after 8–11 min of recruitment-interrupted, zero PEEP ventilation (Fig. 1), thus becoming comparable to oxygenation/shunt fraction in preprone semirecumbent (Table 4). Furthermore, after 14 min of recruitment-interrupted zero PEEP ventilation (Fig. 1), FRC became comparable to the FRC of preprone semirecumbent (Table 4).
PEEP restoration in preprone semirecumbent resulted mainly in full reversal of the changes induced by the preceding PEEP removal in gas exchange. In postprone semirecumbent (Fig. 1) PEEP restoration resulted in a slightly lower shunt fraction than in preprone semirecumbent (Table 4), with PaO2/FIO2 and FRC/EELV remaining substantially closer to preprone semirecumbent rather than prone. Consequently in postprone semirecumbent the employed ventilatory maneuvers and volume history standardization (Fig. 1) adequately changed the initial “prone-like” gas exchange and lung volumes to “preprone semirecumbent-like.”
Regression analysis
Increases in PaO2/FIO2 from preprone semirecumbent to prone either at PEEP or at zero PEEP were closely related to concomitant decreases in LIP pressure of the lung PV curve (R2=0.75–0.79, p<0.001 for all; Fig. 4); similar relationships were observed from prone to postprone semirecumbent (data not shown). Multiple linear regression analysis (dependent variable=postural change in PaO2/FIO2; independent variables=postural changes in PV curve-derived variables and lung volumes) revealed that the sole independent predictor of PaO2/FIO2 increases were the corresponding decreases in the LIP pressure of the lung PV curve. Significant relationships were also observed between increases in PaO2/FIO2 from preprone semirecumbent to prone and concomitant postural changes in PaCO2, FRC, and starting lung compliance (Fig. 5A–C). Postural changes in starting lung compliance were also significantly related with the concomitant increases in FRC (Fig. 5D).
Scatterplots of individual changes in arterial oxygenation (PaO2/FIO2) and changes in the pressure at the lower inflection point (LIP) of the lung pressure-volume curve; the presented PaO2/FIO2 differences are between preprone semirecumbent and prone position and were determined prior to the removal of the positive end-expiratory pressure (A), at zero end-expiratory pressure (B), and after the restoration of the positive end-expiratory pressure (C) (see also text); the curves at either side of the regression lines represent 95% confidence limits
Discussion
The major findings of this study are that in early-stage, severe ARDS (characterized by diffuse lung damage with intra-alveolar and interstitial edema and hyaline membrane) (a) prone position vs. preprone semirecumbent causes leftward shift of the respiratory system and lung PV curves, and reduces LIP pressure and increases UIP volume of the lung PV curve; (b) prone position also increases starting, maximal, and final lung compliance and FRC/EELV, and improves gas exchange; and (c) body posture related changes in PaO2/FIO2 can be explained by concomitant changes in the LIP pressure of the lung PV curve.
Effects of prone position
Our findings on lung PV curve-derived variables suggest augmentation of the continuous [6], lung inflation-induced alveolar recruitment in the prone position, and attenuation of alveolar overdistension above the UIP. Prone ventilation renders lung topography more suitable to recruitment [12] by: (a) providing a more homogeneous distribution of pleural pressures and ventilation [10, 30, 31, 32], (b) relieving regional lung compression by the heart [33] and abdominal contents [34], and (c) improving aeration of dependent lung units [10].
A previous study by Pelosi et al. [10] found no pronation-induced increase in EELV or decrease in shunt fraction. However, they did not employ the criterion of “diffuse ARDS” [16] during patient selection. Also, they enrolled patients with both primary and secondary ARDS [10, 35]. However, patients with differing lung morphologies and/or ARDS may present different physiological responses to PEEP or prone positioning [36, 37].
In the present study oxygenation, PaCO2, shunt fraction, EELV/FRC, and lung compliance were early (within 80 min) and relatively uniformly improved in response to prone positioning (Table 4; Figs. 4, 5). Furthermore, these changes were partly explanatory of each other, thus implying the simultaneous presence of (a) improved ventilation homogeneity leading to both improved ventilation-perfusion matching and effective alveolar ventilation, as suggested by the improved gas exchange (Fig. 5A); (b) an increased FRC-induced improvement in oxygenation (Fig. 5B); and (c) an augmented alveolar recruitment (reflected by the increased starting lung compliance) that induced improvement in both oxygenation (Figs. 4, 5C) and FRC (Fig. 5D). Our results on EELV/FRC and oxygenation/shunt fraction are consistent with those of two recent studies [9, 12], and partly support the notion of “increased EELV-induced improvement in oxygenation” [38].
LIP, PEEP, and oxygenation
With respect to PEEP optimization the significance of the LIP of the respiratory system PV curve is questionable; the LIP may be partly due to the shape of the chest wall PV curve [39]. In such cases the setting of PEEP at “LIP + 2 cmH2O” may not improve oxygenation [39]. In contrast, the LIP of the lung PV curve may more accurately reflect changes in inflation-induced alveolar mechanical behavior, because it is not influenced by the chest wall mechanical abnormalities in ARDS [39]. These concepts are strongly supported by our results, because (a) the LIP pressures of the lung PV curves were different in all body postures, and the contribution of the chest wall PV curve to the respiratory system LIP was considerable in all postures (Table 3; Figs. 2, and 3); (b) the removal and subsequent restoration of PEEP had minor effects on gas exchange in the prone position (Table 4), where the chest wall contribution to the respiratory system LIP was maximized; and (c) the postural change in the LIP pressure of the lung PV curve was the sole independent predictor of the postural change in oxygenation.
Gas exchange, body posture, and PEEP
In prone position PaO2/FIO2 was affected only arithmetically by PEEP. However, the use of PEEP resulted in augmentation of the pronation-induced reduction in shunt fraction, thus being consistent with a recently reported additive effect of PEEP and prone position on true pulmonary shunt [40]. Lastly, the postprone semirecumbent results on gas exchange and lung volumes clearly show that (a) PEEP is necessary for the maintenance of prone position benefits [9], (b) pronation benefits are lost within 14 min of recruitment-interrupted, zero PEEP ventilation, and (c) once lost, these favorable changes cannot be regained by restoring PEEP.
Methodological considerations
The measurements performed in the present study exhibited a limited variability (see T.S1–T.S3 of the Electronic Supplementary Material). A likely source was the manual determination of the pressure-volume coordinates of LIPs and UIPs. The variability in these measurements (also performed with the Measure Tool) most likely resulted in the observed, “roughly stable,” levels of consistency (e.g., 0.24–0.40 cmH2O for pressure measurements; see Electronic Supplementary Material). In our opinion, these consistency limits are acceptable for clinical measurements, thus supporting the combined use of widely available computer programs (SPSS and Photoshop) for PV curve analysis. PV curves were constructed only at zero PEEP, mainly because our objective was to determine the effects of body posture alone (i.e., at zero PEEP conditions) on PV curve variables (e.g., LIP and UIP), according to which ventilator settings could be adjusted [1, 2, 3, 4, 6, 7, 8, 9].
Ethical considerations
In ARDS the prolonged use of zero PEEP is potentially deleterious. In each posture in the present study, the continuous use of zero PEEP was minimized over two consecutive periods of 5 and 9 min, each of which was followed by recruitment maneuver(s) (Fig. 1). Furthermore, despite setting PEEP at 0 cmH2O total PEEP of the respiratory system (determined just prior to PV curve construction) actually ranged within 2.0–5.7 cmH2O. Total PEEP was due to patient intrinsic PEEP at the employed ventilatory settings.
Conclusions
In early, severe, and diffuse ARDS, prone position substantially attenuates the LIP and displaces toward higher inflation volume the UIP of the lung PV curve. The pronation-induced change in the LIP of the lung PV curve explains the postural improvement in arterial oxygenation. The latter is also associated with an increased FRC.
References
Putensen C, Baum M, Hormann C (1993) Selecting ventilator settings according to variables derived from the quasi-static pressure/volume relationship in patients with acute lung injury. Anesth Analg 77:436–447
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Fillho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Harris RS, Hess DR, Venegas JG (2000) An objective analysis of the pressure-volume curve in the acute respiratory distress syndrome. Am J Respir Crit Care Med 161:432–439
Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini JJ, Gattinoni L (2001) Recruitment and derecruitment during acute respiratory failure. A clinical study. Am J Respir Crit Care Med 164:131–140
Albaiceta GM, Taboada F, Parra D, Luyando LH, Calvo J, Menendez R, Otero J (2004) Tomographic study of the pressure-volume curve in acute lung injury. Am J Respir Crit Care Med 170:1066–1072
Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L (1999) Pressure-volume curves and compliance in acute lung injury. Evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med 159:1172–1178
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M (1987) Pressure-volume curve of total respiratory system in acute respiratory failure: computed tomographic scan study. Am Rev Respir Dis 136:730–736
Takeuchi M, Goddon S, Dolhnikoff M, Shimaoka M, Hess D, Amato MB, Kacmarek RM (2002) Set positive end-expiratory pressure during protective ventilation affects lung injury. Anesthesiology 97:682–692
Mentzelopoulos SD, Roussos C, Zakynthinos SG (2005) Prone position reduces lung stress and strain in severe ARDS. Eur Respir J 25:534–544
Pelosi P, Tubiolo D, Mascheroni D, Vicardi P, Crotti S, Valenza P, Gattinoni L (1998) Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med 157:387–393
Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R, the Prone Position Study Group (2001) Effect of prone positioning on the survival of patients with acute respiratory distress syndrome. N Engl J Med 345:568–573
Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L (2003) Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med 167:521–527
Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumelo D, Valenza F, Caironi P, Pesenti A, the Prone-Supine Study Group (2003) Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 31:2727–2733
Guérin C, Badet M, Rosseli S, Heyer L, Sab JM, Langevin B, Philit F, Fournier G, Robert D (1999) Effects of prone position on alveolar recruitment and oxygenation in acute lung injury. Intensive Care Med 25:1222–1230
Ferguson ND, Frutos-Vitar F, Esteban A, Anzueto A, Alia I, Brower RG, Stewart TE, Apezteguia C, Gonzàlez M, Soto L, Abroug F, Brochard L, for the Mechanical Ventilation International Study Group (2005) Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med 33:21–30
Rouby JJ, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P, the CT Scan ARDS Study Group (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome II. Physiological correlations and definition of an ARDS Severity Score. Intensive Care Med 26:1046–1056
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Le Call JR, Morris A, Spragg R, the Consensus Committee (1994) The American-European consensus conference on ARDS. Definition, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126:788–791
Ranieri VΜ, Brienza N, Santostasi S, Puntillo F, Mascia L, Vitale N, Giuliani R, Mermeo V, Bruno F, Fiore T, Brienza A, Slutsky AS (1997) Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome. Role of abdominal distention. Am J Respir Crit Care Med 156:1082–1091
Diehl JL, Lofaso F, Deleuze P, Similowski T, Lemaire F, Brochard L (1994) Clinically relevant diaphragmatic dysfunction after cardiac operations. Ann Thorac Surg 107:487–498
Fernandez R, Blanch L, Artigas A (1993) Inflation static pressure-volume curves of the total respiratory system determined without any instrumentation other than the mechanical ventilator. Intensive Care Med 19:33–38
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Ranieri VM (2002) Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 96:795–802
Mentzelopoulos SD, Zakynthinos SG, Roussos C, Tzoufi MJ, Michalopoulos AS (2003) Prone position improves lung mechanical behavior and enhances gas exchange efficiency in mechanically ventilated chronic obstructive pulmonary disease patients. Anesth Analg 96:1756–1767
Guérin C, Coussa ML, Eissa NT, Corbeil C, Chassé M, Braidy J, Matar N, Milic-Emili J (1993) Lung and chest wall mechanics in mechanically ventilated COPD patients. J Appl Physiol 74:1570–1580
Pelosi P, Croci M, Ravagnan I, Cerisara M, Vicardi P, Lissoni A, Gattinoni L (1997) Respiratory system mechanics in sedated, paralyzed, morbidly obese patients. J Appl Physiol 83:811–818
Lee WL, Stewart TE, MacDonald R, Lapinsky S, Banayan D, Hallett D, Mehta S (2002) Safety of pressure-volume curve measurement in acute lung injury and ARDS using a syringe technique. Chest 121:1595–1601
Milic-Emili J, Mead J, Turner JM (1964) Topography of esophageal pressure as a function of posture in man. J Appl Physiol 19:212–216
Pelosi P, Croci M, Calappi E, Cerisara M, Mulazzi D, Vicardi P, Gattinoni L (1995) The prone positioning during general anesthesia minimally affects respiratory mechanics while improving functional residual capacity and increasing oxygen tension. Anesth Analg 80:955–960
Pelosi P, Croci M, Callapi E, Mulazzi D, Cerisara M, Vercesi P, Vicardi P, Gattinoni L (1996) Prone positioning improves pulmonary function in obese patients during general anesthesia. Anesth Analg 83:578–583
Mutoh T, Guest RJ, Lamm WJE, Albert RK (1992) Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis 146:300–306
Yang QH, Kaplowitz MR, Lai-Fook SJ (1988) Regional variations in lung expansion in rabbits: prone vs. supine positions. J Appl Physiol 67:1371–1376
Olson LE, Lai-Fook SJ (1988) Pleural liquid pressure measured with rib capsules in anesthetized ponies. J Appl Physiol 64:102–107
Albert RK, Hubmayr RD (2000) The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med 161:1660–1665
Rouby JJ, Puybasset L, Niezkowska A, Lu Q (2003) Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med 31 [Suppl 4]:S285–S295
Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A (1998) Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med 158:3–11
Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. Intensive Care Med 26:1215–1227
Pelosi P, Brazzi L, Gattinoni L (2002) Prone position in acute respiratory distress syndrome. Eur Respir J 20:1017–1028
Douglas WW, Rheder K, Beynen FM, Sessler AD, Marsh HM (1977) Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis 115:559–566
Mergoni M, Martelli A, Volpi A, Primavera S, Zuccoli P, Rossi A (1997) Impact of positive end-expiratory pressure on chest wall and lung pressure-volume curve in acute respiratory failure. Am J Respir Crit Care Med 156:846–854
Gainnier M, Michelet P, Thirion X, Arnal JM, Sainty JM, Papazian L (2003) Prone position and positive end-expiratory pressure in acute respiratory distress syndrome. Crit Care Med 31:2719–2726
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was funded solely by the Department of Intensive Care Medicine of Evaggelismos General Hospital, Athens, Greece
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Mentzelopoulos, S.D., Roussos, C. & Zakynthinos, S.G. Static pressure volume curves and body posture in acute respiratory failure. Intensive Care Med 31, 1683–1692 (2005). https://doi.org/10.1007/s00134-005-2838-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2838-3