Abstract
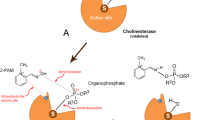
The inhibition of cholinesterase (ChE) activity has been used as a biomarker of exposure to organophosphate and carbamate insecticides. ChE of nesting female green turtles (Chelonia mydas) were biochemically characterized using two substrates, acetylthiocholine iodide and butyrylthiocholine iodide, and three ChE inhibitors (eserine sulfate, BW284C51 and iso-OMPA). The results indicated that BChE is the predominant plasma ChE in female C. mydas, but with atypical properties that differ from those found in human BChE. Eggs from green turtles nesting at two sites in Laguna de Terminos contained µg g−1 (wet weight) quantities of organochlorine (OC) pesticides. Drins (aldrin, dieldrin, endrin, endrin ketone, endrin aldehyde) were found at the highest concentrations with no significant differences in the concentrations in eggs collected at the two sampling sites. A negative relationship was found between levels of OC pesticides in eggs and BChE activity in the plasma of female turtles laying the eggs. Since OC pesticides are not cholinesterase inhibitors, we hypothesized that this inverse relationship may be related to an antagonistic effect between OCs and organophosphate pesticides and mobilization of OCs from the fatty tissues of the female turtles into their eggs. However, further study is required to verify the hypothesis. It is also possible that other contaminants, such as petroleum hydrocarbons are responsible for the modulation of cholinesterase activity in female turtles.



Similar content being viewed by others
References
Bain D, Buttemer WA, Astheimer L, Fildes K, Hooper MJ (2004) Effects of sublethal fenitrothion ingestion on cholinesterase inhibition, standard metabolism, thermal preference, and prey capture ability in the Australian central bearded dragon (Pogona vitticeps: Agamidae). Environ Toxicol Chem 23(1):109–116
Bocquené G, Galgani F, Truquet P (1990) Characterization and assay conditions for use of AChE activity from several marine species in pollution monitoring. Mar Environ Res 30(2):75–89
Carvalho FP, Villeneuve JP, Cattini C, Rendón J, Mota de Oliveira J (2009) Pesticide and PCB residues in the aquatic ecosystems of Laguna de Terminos, a protected area of the coast of Campeche, México. Chemosphere 74(7):988–995
Chi-Coyoc T, Segura GE, Vallarino Moncada G, Contreras JA, Vela GE, Reyna JL (2016) Organochlorine and anticholinergic pesticides in wild mice from wetland ecosystems of the Gulf of Mexico. THERYA 7(3):465–482
Cohen SD, Murphy SD (1974) A simplified bioassay for organophosphate detoxication and interactions. Toxicol Appl Pharmacol 27(3):537–550
EPA (2007a) Method 3550C. Ultrasonic extraction. Revision 3
EPA (2007b) Method 3535ª. Solid-phase extraction. Revision 1
EPA (2007c) Method 1699: Pesticides in water, soil, sediment, biosolids, and tissue by HRGC/HRMS
Flammarion P, Noury P, Garric C (2002) The measurement of cholinesterase activities as a biomarker in chub (Leuciscus cephalus): the fish length should not be ignored. Environ Pollut 120(2):325–330
Gold-Bouchot G, Zapata-Pérez O, Ceja-Moreno V, Rodríguez-Fuentes G, Simá-Alvarez R, Aguirre-Macedo ML, Vidal-Martínez VM, Da Ros L, Nasci C (2007) Biological effects of environmental pollutants in American oyster, Crassostrea virginica: a field study in Laguna de Terminos, Mexico. Int J Environ Health 1(2):171–184
Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA et al (2010) Global research priorities for sea turtles: informing management and conservation in the 21st century. Endanger Species Res 11(3):245–269
INE/SEMARNAP (Instituto Nacional de Ecología/Secretaría del Medio Ambiente Recursos Naturales y Pesca) (1997) Programa de manejo del área de protección de flora y fauna “Laguna de Términos”. Mexico. Instituto Nacional de Ecología, México, p 167
O’Brien RD (1969) Insecticides, action and metabolism, 2nd edn. Academic Press, New York, p 346
Owens DW, Ruiz GJ (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 36(1):1–20
Rodríguez-Fuentes G, Armstrong J, Schlenk D (2008) Characterization of muscle cholinesterases from two demersal flatfish collected near a municipal wastewater outfall in southern California. Ecotoxicol Environ Saf 69(3):466–471
Sanchez-Hernandez JC (2001) Wildlife exposure to organophosphorus insecticides. Rev Environ Contam Toxicol 172:21–63
Sanchez-Hernandez JC, Sanchez BM (2002) Lizard cholinesterases as biomarkers of pesticide exposure: enzymological characterization. Environ Toxicol Chem 21(11):2319–2325
Schmidt SR (2003) Reptile cholinesterase characterization and use in monitoring anticholinesterases. Thesis in Environmental Toxicology. Texas Tech University
Seminoff JA (Southwest Fisheries Science Center, U.S.) (2004) Chelonia mydas. The IUCN Red List of Threatened Species 2004: e.T4615A11037468
Sturm A, de Assis HCD, Hansen PD (1999) Cholinesterases of marine teleost fish: enzymological characterization and potential use in the monitoring of neurotoxic contamination. Mar Environ Res 47(4):389–398
Triolo A, Coon JM (1966) The protective effect of aldrin against the toxicity of organophosphate anticholinesterases. J Pharmacol Exp Ther 154(3):613–623
van de Merwe JP, Hodge M, Olszowy HA, Whittier J, Ibrahim K, Lee SY (2010) Using blood samples to estimate persistent organic pollutants and metals in green sea turtles (Chelonia mydas). Mar Pollut Bull 60(4):579–588
Acknowledgements
Special thanks to Dr. Ann Grant for comments on an earlier draft that improved the quality of our manuscript. Author thanks financial support from UNAM-PAPIIT-DGAPA grants IA200214 and IA202416 and the Universidad Autónoma del Carmen. We thank the Turtle Station Managers, Biol. Vicente Guzmán Hernández and Ing. Alfonso Díaz Molina, for the support given during the sample collection. Special thanks to L.E.F.D. Félix Canul Cejas for his guidance and advice during the blood collection. Thanks to Andrés Cruz Quintana for his assistance during the collection of all blood samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivas-Hernández, G., May-Uc, Y., Noreña-Barroso, E. et al. Plasma Cholinesterase Activity in Female Green Turtles Chelonia mydas Nesting in Laguna de Terminos, Mexico Related to Organochlorine Pesticides in Their Eggs. Bull Environ Contam Toxicol 100, 101–105 (2018). https://doi.org/10.1007/s00128-017-2250-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2250-z