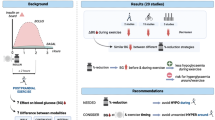
Abstract
Recent guidelines suggest that adding anaerobic (high intensity or resistance) activity to an exercise session can prevent blood glucose declines that occur during aerobic exercise in individuals with type 1 diabetes. This theory evolved from earlier study data showing that sustained, anaerobic activity (high intensity cycling) increases blood glucose levels in these participants. However, studies involving protocols where anaerobic (high intensity interval) and aerobic exercise are combined have extremely variable glycaemic outcomes, as do resistance exercise studies. Scrutinising earlier studies will reveal that, in addition to high intensity activity (intervals or weight lifting), these protocols had another common feature: participants were performing exercise after an overnight fast. Based on these findings, and data from recent exercise studies, it can be argued that participant prandial state may be a more dominant factor than exercise intensity where glycaemic changes in individuals with type 1 diabetes are concerned. As such, a reassessment of study outcomes and an update to exercise recommendations for those with type 1 diabetes may be warranted.
Graphical abstract

Similar content being viewed by others
Introduction
Fear of hypoglycaemia is a major barrier to physical activity among individuals with type 1 diabetes [1]. As such, most research to date has examined the acute glycaemic effects of different activity types to refine hypoglycaemia prevention guidelines. Most studies have focused on activity intensity and duration, in addition to its timing relative to meals, snacks and insulin adjustments.
Recently, interest in anaerobic (i.e. high intensity) exercise has increased due to its association with smaller declines in blood glucose and potentially fewer occurrences of hypoglycaemia. Anaerobic exercise is defined as intense, short-duration physical activity, where the fuels provided for the contracting muscles are independent of oxygen availability [2]. Weight lifting, sprinting and sustained high intensity (generally >70–80% of peak aerobic capacity [V̇O2peak) cycling, running, swimming, etc. are all considered ‘anaerobic’. As these activities cannot be sustained for long durations, they are often combined with periods of either low intensity aerobic activity or resting recovery. These exercise sessions are described as high intensity interval exercise (HIIE).
Since the 1980s, researchers examining exercise in the context of type 1 diabetes have generally believed that high intensity (anaerobic) activities increase blood glucose levels while aerobic exercise is associated with blood glucose declines. Indeed, even the most recent literature reviews and consensus statements around exercise/physical activity and type 1 diabetes make such statements [3, 4]. Many recommendations about starting blood glucose targets and insulin adjustments for exercise are also based on these foundations [5]. Is it possible, however, that these ideas are rooted in a decades-long misinterpretation of data?
Many studies of anaerobic exercise were undertaken in the morning, after an overnight fast. The decision to have participants perform these protocols while fasting was probably taken to avoid the complications of making insulin adjustments before exercise, and indeed to decrease the risk of hypoglycaemia from elevated insulin levels. Early morning, before the first meal of the day, is the only time that individuals are likely to be in a truly fasted, or post-absorptive, state. Most of the day is spent in a postprandial state, where remnants of the last meal may enter the blood stream as late as 4 to 6 h after it was consumed. In individuals with type 1 diabetes, it is also likely that some of the prandial insulin taken with that meal will still be circulating. In the fasted state, however, in addition to lower insulin levels, fuel selection both at rest and during exercise is very different, which may have had an unintended impact on blood glucose outcomes.
Sustained high intensity exercise
Several early studies of anaerobic activity in individuals with type 1 diabetes demonstrated clear blood glucose increases (and subsequent hyperglycaemia) following sustained (~10–12 min), high intensity (≥80% of V̇O2peak, or incremental tests to exhaustion) exercise. Mitchell et al [6], observed an increase (from 4.8 ± 0.2 to 7.1 ± 0.4 mmol/l) in blood glucose levels throughout exercise and up to 2 h following exercise when six (four female/two male) participants with type 1 diabetes performed sustained high intensity (80% V̇O2peak) cycling until exhaustion (10 ± 2 min). Similarly, when six adult male participants with type 1 diabetes performed an incremental workload test to exhaustion (~12–14 min), Purdon et al [7] measured an increase in blood glucose levels from 4.8 ± 0.2 mmol/l pre-exercise to a 2-min post-exercise peak of 7.9 ± 0.5 mmol/l. Blood glucose levels did not return to baseline after 2 h of recovery. Sigal et al [8] measured a comparable glucose increase (+2.65 ± 0.32 mmol/l) from the start of exercise to the glycaemic peak 4 min into recovery, when six young, fit male participants with type 1 diabetes performed sustained high intensity (89–98% V̇O2peak) cycle ergometer exercise (12.3 ± 0.7 min). In all three studies, participants had very good glycaemic management (close to normal HbA1c). Importantly, another design element common to all three studies (in addition to the sustained, anaerobic exercise) is that they were performed after an overnight fast.
Over the past 5 years, we have performed similar incremental workload tests to exhaustion for V̇O2peak tests in our lab on 34 individuals with type 1 diabetes (25 female; aged 29.2 ± 8.3 years; mean V̇O2peak=38.7 ± 7.7 ml kg-1 min-1; HbA1c=61 ± 3 mmol/mol [7.7 ± 1.0%]). The mean test duration was similar to those listed above (9.7 ± 3.0 min). All tests were performed within 3 to 5 h of consuming a meal. In stark contrast to the studies described above, the mean blood glucose change was a decrease (−1.0 ± 2.3 mmol/l, p=0.03) from the start to the end of exercise. More participants (n=21) experienced declining blood glucose than those who experienced an increase (n=13) (Fig. 1). While it can be argued that age, sex or physical fitness could have affected blood glucose outcomes [9], there was no consistent effect of these characteristics when assessed with regression analyses.
Resistance exercise
Resistance exercise, generally consisting of short bouts of relatively forceful muscle contraction, is also an anaerobic activity. The acute glycaemic effects of this type of activity in individuals with type 1 diabetes has only been studied in the context of weight lifting, and has generally been limited to one type of protocol (a moderate/mass-building protocol involving three sets of eight repetitions) [10,11,12,13,14]. In 2013, Yardley et al [11], observed a blood glucose decrease from 8.4 ± 2.7 mmol/l to 6.8 ± 2.3 mmol/l (p=0.008) when 12 participants with type 1 diabetes (two female; aged 31.8 ± 15.3 years; V̇O2peak=51.2 ± 10.8; HbA1c=54 ± 0.3 mmol/mol [7.1 ± 1.1%]) performed three sets of eight repetitions of seven exercises targeting all major muscle groups. Exercises were performed at the participants’ eight repetition maximum (8RM—the maximum amount of weight that could be safely lifted with good form eight times). Using a very similar protocol (three sets of 8 to 12 repetitions at 60–80% of 1RM, targeting all major muscle groups), ten participants in a study by Reddy et al [10] [six female; age 33 ± 6 years; HbA1c=57 ± 0.3 mmol/mol [7.4 ± 1.0%]) experienced a similar blood glucose decline (−1.33 ± 1.78 mmol/l, p=0.007). Both protocols involved a 90 s rest between sets, were performed roughly 4 to 5 h after lunch, and incorporated insulin adjustments alongside a pre-exercise snack to prevent blood glucose declines.
In contrast, however, four separate publications by Turner et al [12,13,14,15] reported rising blood glucose levels with resistance exercise, despite altering the number of sets of exercise performed [15] and the intensity (i.e. resistance and number of repetitions) of the sets [14]. Importantly, an almost identical protocol to that used by both Reddy et al [10] and Yardley et al [11] (three sets of ten repetitions at ~64% of the individual’s 1RM) also increased blood glucose levels in eight participants (one female; aged 38 ± 6 years; HbA1c=72 ± 0.3 mmol/mol [8.7 ± 1.0%]) with type 1 diabetes [13]. The participants were notably similar across studies: there were slightly more female than male participants (except Yardley et al [11]), all of whom were recreationally active, having managed type 1 diabetes for more than a decade on average, and a similar mean age. As such, it is unlikely that age, sex or physical fitness affected the results [9]. It is more likely that one key aspect of study design was responsible for the differences observed: data collected by Turner et al [12,13,14,15] involved participants exercising after an overnight fast, while studies by Reddy et al [10] and Yardley et al [11] started their exercise protocols between 16:00 and 17:00 hours with participants having consumed a meal in the previous 4–5 h.
HIIE
If it can be believed that sustained high intensity exercise consistently increases blood glucose levels in individuals with type 1 diabetes, then logic would dictate that performing this type of activity intermittently with periods of rest or low intensity aerobic recovery between them should lead to relatively stable blood glucose levels during exercise. Indeed, HIIE has been associated with a lower risk of hypoglycaemia during exercise compared with aerobic exercise [16,17,18,19,20,21,22,23,24], although it may increase the risk of post-exercise hypoglycaemia [25]. However, glycaemic outcomes from HIIE studies involving participants with type 1 diabetes have been very inconsistent, with some showing no change [17, 18] or increasing blood glucose [16], with others showing non-significant [23, 24] or significant [19,20,21, 26] blood glucose declines by the end of exercise. Participant characteristics such as age, sex and physical fitness may play some role in the variable responses [9], as could discrepancies among the exercise protocols themselves (interval intensities between 85% V̇O2peak and supramaximal, interval length between 4 and 30 s). However, the only thing consistent about the studies where blood glucose declined is that they were all performed 3 to 5 h after a meal [19,20,21, 23, 24, 26].
Fasting vs fed repeated measures studies
A handful of small studies, using repeated measures study designs, have compared fasted vs fed exercise using a single group of participants with type 1 diabetes within each study. Where anaerobic exercise is concerned, two separate studies (both n=12) showed clear blood glucose decreases with afternoon resistance [27] and HIIE [28] (performed ~4–5 h after a meal), while the same protocols performed by the same participants after an overnight fast resulted in an increase or no change in blood glucose, respectively. Even aerobic exercise, thought to consistently decrease blood glucose levels [3, 4], can increase blood glucose when performed under these conditions [29, 30]. A small study by Ruegemer et al [29] (n=6; three female participants; age 30 ± 4 years; HbA1c=58 ± 0.2 mmol/mol [7.5 ± 0.6%]) measured an increase in blood glucose (6.7 ± 0.4 mmol/l to 9.1 ± 0.4 mmol/l) over 30 min of moderate (60% V̇O2peak) aerobic exercise performed after an overnight fast, which was absent when participants performed the same protocol at 16:00 hours (4 h after lunch) on a different day. Similarly, Yamanouchi et al [30] found a blood glucose decline (15.3 ± 3.0 to 11.0 ± 0.7 mmol/l) during a walking protocol 2 h after breakfast (09:00 hours), when the same protocol at roughly the same time of day (07:00 hours) was associated with no blood glucose decline when performed before breakfast. Overall, the trend from these studies is clear: on average, blood glucose does not decline, and will generally increase when exercise is performed after an overnight fast, regardless of exercise intensity.
Fasted exercise metabolism in type 1 diabetes
There are several explanations for the observed glycaemic trends. The first, and easiest, explanation is that circulating insulin levels are lower after an overnight fast than they are mid- to late afternoon. However, this on its own would not explain rising blood glucose levels regardless of exercise modality. An alternate explanation involves lipids being prioritised as a fuel source when the body is in a fasted state [31]. As triglycerides are metabolised, the resulting glycerol could act as a gluconeogenic precursor, thereby increasing blood glucose. The resulting increase in circulating NEFA from lipolysis (which are higher after fasted exercise) [32] could also temporarily increase insulin resistance [33], which would account for rising blood glucose levels during exercise, and persistent hyperglycaemia [16, 27] after exercise, when performed after an overnight fast.
High growth hormone levels have been associated with the ‘dawn phenomenon’, an early morning blood glucose surge experienced by individuals with type 1 diabetes [34, 35]. While this phenomenon itself may be responsible for some of the higher glucose levels experienced with fasted exercise, the underlying exercise-induced growth hormone elevations (which are higher with greater intensity [36], and enhanced by fasting [37]) may play a role in increasing blood glucose levels with exercise intensity and duration. In a previous study of individuals with type 1 diabetes, higher growth hormone was associated with glucose sparing [38] during exercise late in the day and may be playing a greater role when the activity is performed after an overnight fast.
Are these responses universal?
Anyone with type 1 diabetes who is physically active, and anyone who has involved individuals with type 1 diabetes in exercise studies, knows that blood glucose responses to exercise are extremely variable. Most of the trends discussed above are the mean changes in blood glucose in a group of participants. Data from non-fasted incremental V̇O2peak tests performed in our lab over the past 5 years (n=34) produced blood glucose changes ranging from −5.4 mmol/l to +3.5 mmol/l (Fig. 1). There were no clear differences between those using multiple daily injections of insulin vs those using insulin pumps, between male and female participants, between higher fitness (V̇O2peak >40 ml O2 kg-1 min-1) and lower fitness, or between younger (<30 years) and older individuals. It is likely that a combination of factors, including the timing of previous insulin injections, dictate whether glucose increases or decreases during one of these tests. However, it should be noted that the mean blood glucose change over all of these tests (performed 3–5 h after a meal) was −1.0 ± 2.3 mmol/l (p=0.03), rather than the increase one would expect if sustained high intensity exercise had a consistent hyperglycaemic response. These findings are consistent with a large observational study (n=5157) where self-reported data indicated a decrease in blood glucose levels in the majority of participants (75.8%) during exercise, regardless of the type or intensity [39].
As can be seen in Fig. 2, changes in glucose during fasted resistance exercise (Fig. 2a [methods described elsewhere [27]]) and fasted HIIE (Fig. 2b [methods described elsewhere [28]]), show that most participants experience rising blood glucose levels during fasted anaerobic exercise (9 out of 12 and 10 out of 12, respectively). Riddell et al [16] also noted this response among 16 participants with type 1 diabetes (four female; aged 34.7 ± 10.3 years; HbA1c=54 ± 0.3 mmol/mol [7.1 ± 0.8%]) performing a combination of interval cycling and multimodal training during a 25 min exercise session after an overnight fast. Where each participant performed the same session four times, increasing glucose was observed in 62 of the 64 exercise sessions.
So what?
Current exercise safety guidelines for individuals with type 1 diabetes suggest that those performing anaerobic activities can start with lower blood glucose levels [3]. Some even suggest that bolus insulin before exercise may be appropriate [5]. These recommendations are based on the assumption that anaerobic activities cause blood glucose levels to rise during exercise. However, from the literature available, this only seems to occur somewhat consistently when exercising after an overnight fast. While seasoned athletes with type 1 diabetes will generally have learned to manage their blood glucose levels accordingly, these recommendations could be misleading and may cause hypoglycaemia for less experienced exercisers. Conversely, the aggressive insulin reductions currently suggested for aerobic exercise may be unnecessary (and may actually cause hyperglycaemia) if the activity is performed after an overnight fast, provided that it is not prolonged (i.e. >45 min) [40]. In light of this information, future guidelines and consensus statements for exercise and physical activity in individuals with type 1 diabetes should consider providing different advice based on participant prandial status. Furthermore, for individuals whose fear of hypoglycaemia is a barrier to exercise and physical activity, the data suggest that exercising after an overnight fast may be a safer option.
Abbreviations
- HIIE:
-
High intensity interval exercise
- RM:
-
Repetition maximum
- V̇O2peak :
-
Peak aerobic capacity
References
Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H (2008) Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 31(11):2108–2109. https://doi.org/10.2337/dc08-0720
ACSM (2021) ACSM’s Guidelines for Exercise Testing and Prescription, Eleventh edn. Wolters Kluwer, Philadelphia, PA, USA
Riddell MC, Gallen IW, Smart CE et al (2017) Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 5(5):377–390. https://doi.org/10.1016/S2213-8587(17)30014-1
Colberg SR, Sigal RJ, Yardley JE et al (2016) Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 39(11):2065–2079. https://doi.org/10.2337/dc16-1728
Moser O, Riddell MC, Eckstein ML et al (2020) Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 63(12):2501–2520. https://doi.org/10.1007/s00125-020-05263-9
Mitchell TH, Abraham G, Schiffrin A, Leiter LA, Marliss EB (1988) Hyperglycemia after intense exercise in IDDM subjects during continuous subcutaneous insulin infusion. Diabetes Care 11(4):311–317. https://doi.org/10.2337/diacare.11.4.311
Purdon C, Brousson M, Nyveen SL et al (1993) The roles of insulin and catecholamines in the glucoregulatory response during intense exercise and early recovery in insulin-dependent diabetic and control subjects. J Clin Endocrinol Metab 76(3):566–573. https://doi.org/10.1210/jcem.76.3.8445012
Sigal RJ, Purdon C, Fisher SJ, Halter JB, Vranic M, Marliss EB (1994) Hyperinsulinemia prevents prolonged hyperglycemia after intense exercise in insulin-dependent diabetic subjects. J Clin Endocrinol Metab 79(4):1049–1057. https://doi.org/10.1210/jcem.79.4.7962273
Yardley JE, Brockman NK, Bracken RM (2018) Could Age, Sex and Physical Fitness Affect Blood Glucose Responses to Exercise in Type 1 Diabetes? Front Endocrinol (Lausanne) 9:674. https://doi.org/10.3389/fendo.2018.00674
Reddy R, Wittenberg A, Castle JR et al (2019) Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can J Diabetes 43(6):406–414 e401. https://doi.org/10.1016/j.jcjd.2018.08.193
Yardley JE, Kenny GP, Perkins BA et al (2013) Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care 36(3):537–542. https://doi.org/10.2337/dc12-0963
Turner D, Luzio S, Gray BJ et al (2016) Algorithm that delivers an individualized rapid-acting insulin dose after morning resistance exercise counters post-exercise hyperglycaemia in people with Type 1 diabetes. Diabet Med 33(4):506–510. https://doi.org/10.1111/dme.12870
Turner D, Luzio S, Kilduff LP et al (2014) Reductions in resistance exercise-induced hyperglycaemic episodes are associated with circulating interleukin-6 in type 1 diabetes. Diabet Med 31(8):1009–1013. https://doi.org/10.1111/dme.12462
Turner D, Gray BJ, Luzio S et al (2016) Similar magnitude of post-exercise hyperglycemia despite manipulating resistance exercise intensity in type 1 diabetes individuals. Scand J Med Sci Sports 26(4):404–412. https://doi.org/10.1111/sms.12472
Turner D, Luzio S, Gray BJ et al (2015) Impact of single and multiple sets of resistance exercise in type 1 diabetes. Scand J Med Sci Sports 25(1):e99–109. https://doi.org/10.1111/sms.12202
Riddell MC, Pooni R, Yavelberg L et al (2019) Reproducibility in the cardiometabolic responses to high-intensity interval exercise in adults with type 1 diabetes. Diabetes Res Clin Pract 148:137–143. https://doi.org/10.1016/j.diabres.2019.01.003
Soon WHK, Guelfi KJ, Davis EA, Smith GJ, Jones TW, Fournier PA (2019) Effect of combining pre-exercise carbohydrate intake and repeated short sprints on the blood glucose response to moderate-intensity exercise in young individuals with Type 1 diabetes. Diabet Med 36(5):612–619. https://doi.org/10.1111/dme.13914
Scott SN, Cocks M, Andrews RC et al (2019) Fasted High-Intensity Interval and Moderate-Intensity Exercise Do Not Lead to Detrimental 24-Hour Blood Glucose Profiles. J Clin Endocrinol Metab 104(1):111–117. https://doi.org/10.1210/jc.2018-01308
Guelfi KJ, Jones TW, Fournier PA (2005) The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 28(6):1289–1294. https://doi.org/10.2337/diacare.28.6.1289
Iscoe KE, Riddell MC (2011) Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with Type 1 diabetes mellitus. Diabet Med 28(7):824–832. https://doi.org/10.1111/j.1464-5491.2011.03274.x
Maran A, Pavan P, Bonsembiante B et al (2010) Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with Type 1 diabetes. Diabetes Technol Ther 12(10):763–768. https://doi.org/10.1089/dia.2010.0038
Campbell MD, West DJ, Bain SC et al (2015) Simulated games activity vs continuous running exercise: a novel comparison of the glycemic and metabolic responses in T1DM patients. Scand J Med Sci Sports 25(2):216–222. https://doi.org/10.1111/sms.12192
Moser O, Tschakert G, Mueller A et al (2015) Effects of High-Intensity Interval Exercise versus Moderate Continuous Exercise on Glucose Homeostasis and Hormone Response in Patients with Type 1 Diabetes Mellitus Using Novel Ultra-Long-Acting Insulin. PLoS One 10(8):e0136489. https://doi.org/10.1371/journal.pone.0136489
Dube MC, Lavoie C, Weisnagel SJ (2013) Glucose or intermittent high-intensity exercise in glargine/glulisine users with T1DM. Med Sci Sports Exerc 45(1):3–7. https://doi.org/10.1249/MSS.0b013e31826c6ad3
Valli G, Minnock D, Tarantino G, Neville RD (2021) Delayed effect of different exercise modalities on glycaemic control in type 1 diabetes mellitus: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 31(3):705–716. https://doi.org/10.1016/j.numecd.2020.12.006
Campbell MD, Walker M, Bracken RM et al (2015) Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care 3(1):e000085. https://doi.org/10.1136/bmjdrc-2015-000085
Toghi-Eshghi SR, Yardley JE (2019) Morning (Fasting) vs Afternoon Resistance Exercise in Individuals With Type 1 Diabetes: A Randomized Crossover Study. J Clin Endocrinol Metab 104(11):5217–5224. https://doi.org/10.1210/jc.2018-02384
Yardley JE (2020) Fasting May Alter Blood Glucose Responses to High-Intensity Interval Exercise in Adults With Type 1 Diabetes: A Randomized, Acute Crossover Study. Can J Diabetes 44(8):727–733. https://doi.org/10.1016/j.jcjd.2020.09.007
Ruegemer JJ, Squires RW, Marsh HM et al (1990) Differences between prebreakfast and late afternoon glycemic responses to exercise in IDDM patients. Diabetes Care 13(2):104–110. https://doi.org/10.2337/diacare.13.2.104
Yamanouchi K, Abe R, Takeda A, Atsumi Y, Shichiri M, Sato Y (2002) The effect of walking before and after breakfast on blood glucose levels in patients with type 1 diabetes treated with intensive insulin therapy. Diabetes Res Clin Pract 58(1):11–18. https://doi.org/10.1016/s0168-8227(02)00099-2
Vieira AF, Costa RR, Macedo RC, Coconcelli L, Kruel LF (2016) Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. Br J Nutr 116(7):1153–1164. https://doi.org/10.1017/S0007114516003160
Aird TP, Davies RW, Carson BP (2018) Effects of fasted vs fed-state exercise on performance and post-exercise metabolism: A systematic review and meta-analysis. Scand J Med Sci Sports 28(5):1476–1493. https://doi.org/10.1111/sms.13054
Bajaj M, Berria R, Pratipanawatr T et al (2002) Free fatty acid-induced peripheral insulin resistance augments splanchnic glucose uptake in healthy humans. Am J Physiol Endocrinol Metab 283(2):E346–E352. https://doi.org/10.1152/ajpendo.00329.2001
Campbell PJ, Bolli GB, Cryer PE, Gerich JE (1985) Sequence of events during development of the dawn phenomenon in insulin-dependent diabetes mellitus. Metabolism 34(12):1100–1104. https://doi.org/10.1016/0026-0495(85)90153-2
Edge JA, Matthews DR, Dunger DB (1990) The dawn phenomenon is related to overnight growth hormone release in adolescent diabetics. Clin Endocrinol 33(6):729–737. https://doi.org/10.1111/j.1365-2265.1990.tb03910.x
Pritzlaff CJ, Wideman L, Weltman JY et al (1999) Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol 87(2):498–504. https://doi.org/10.1152/jappl.1999.87.2.498
Vendelbo MH, Christensen B, Gronbaek SB et al (2015) GH signaling in human adipose and muscle tissue during ‘feast and famine’: amplification of exercise stimulation following fasting compared to glucose administration. Eur J Endocrinol 173(3):283–290. https://doi.org/10.1530/EJE-14-1157
Yardley JE, Kenny GP, Perkins BA et al (2012) Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 35(4):669–675. https://doi.org/10.2337/dc11-1844
Colberg SR, Hernandez MJ, Shahzad F (2013) Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care 36(10):e177. https://doi.org/10.2337/dc13-0965
McGaugh SM, Zaharieva DP, Pooni R et al (2021) Carbohydrate Requirements for Prolonged, Fasted Exercise With and Without Basal Rate Reductions in Adults With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Care 44(2):610–613. https://doi.org/10.2337/dc20-1554
Acknowledgements
The author thanks Mr Corbin Nitz (Augustana Faculty, University of Alberta, Canada) for his help in editing the manuscript and Ms Saru Toor (Faculty of Science, University of Alberta, Canada) for developing the graphical abstract.
Authors’ relationships and activities
JEY has received in kind support from Dexcom Inc, Abbott and LifeScan Canada, and Speaker’s fees from Dexcom and Abbott.
Contribution statement
JEY is the sole author of this work.
Funding
JEY is supported by an Alberta New Investigator Award from the Heart and Stroke Foundation of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yardley, J.E. Reassessing the evidence: prandial state dictates glycaemic responses to exercise in individuals with type 1 diabetes to a greater extent than intensity. Diabetologia 65, 1994–1999 (2022). https://doi.org/10.1007/s00125-022-05781-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05781-8