Abstract
Aims/hypothesis
N-terminal pro-B-type natriuretic peptide (NT-proBNP) is the gold standard prognostic biomarker for diagnosis and occurrence of heart failure. Here, we compared its prognostic value for the occurrence of congestive heart failure with that of plasma mid-region pro-adrenomedullin (MR-proADM), a surrogate for adrenomedullin, a vasoactive peptide with vasodilator and natriuretic properties, in people with type 2 diabetes.
Methods
Plasma MR-proADM concentration was measured in baseline samples of a hospital-based cohort of consecutively recruited participants with type 2 diabetes. Our primary endpoint was heart failure requiring hospitalisation.
Results
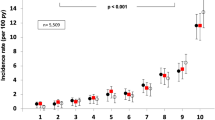
We included 1438 participants (age 65 ± 11 years; 604 women and 834 men). Hospitalisation for heart failure occurred during follow-up (median 64 months) in 206 participants; the incidence rate of heart failure was 2.5 (95% CI 2.2, 2.9) per 100 person-years. Plasma concentrations of MR-proADM and NT-proBNP were significantly associated with heart failure in a Cox multivariable analysis model when adjusted for age, diabetes duration, history of coronary heart disease, proteinuria and baseline eGFR (adjHR [95%CI] 1.83 [1.51, 2.21] and 2.20 [1.86, 2.61], respectively, per 1 SD log10 increment, both p < 0.001). MR-proADM contributed significant supplementary information to the prognosis of heart failure when we considered the clinical risk factors (integrated discrimination improvement [IDI, mean ± SEM] 0.021 ± 0.007, p = 0.001) (Table 3). Inclusion of NT-proBNP in the multivariable model including MR-proADM contributed significant complementary information on prediction of heart failure (IDI [mean ± SEM] 0.028 ± 0.008, p < 0.001). By contrast, MR-proADM did not contribute supplementary information on prediction of heart failure in a model including NT-proBNP (IDI [mean ± SEM] 0.003 ± 0.003, p = 0.27), with similar results for heart failure with reduced ejection fraction and preserved ejection fraction.
Conclusions/interpretation
MR-proADM is a prognostic biomarker for heart failure in people with type 2 diabetes but gives no significant complementary information on prediction of heart failure compared with NT-proBNP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Cardiovascular diseases are a leading cause of mortality in people with diabetes [1]. Compared with non-diabetic individuals, diabetic individuals have a higher risk of developing coronary heart disease and cardiomyopathy, pathologies that predispose to chronic heart failure and contribute to the higher cardiovascular mortality observed in people with diabetes [2]. Congestive heart failure (CHF) is the most frequent cause of hospitalisation for cardiovascular disease in individuals over 65 years of age [3] and is a major public health problem with a negative impact on the individual’s quality of life. Its incidence is higher in people with diabetes than in the general population [4,5,6,7,8].
Adrenomedullin (ADM) is a 52 amino acid peptide expressed and secreted in many cell types including endothelial and vascular smooth muscle cells, cardiomyocytes, fibroblasts, monocytes and leucocytes [9]. Production and secretion of ADM is increased in response to hypoxia and ischaemia [10]. At physiological concentration, ADM has vasodilatory and hypotensive effects in the systemic and pulmonary circulations, as well as a natriuretic effect [11]. ADM and mid-region proADM (MR-proADM) are formed in equimolar amounts from a precursor peptide, pre-proADM, by enzymatic cleavage. MR-proADM is easily measurable in blood samples and is a stable surrogate marker of ADM [12]. High plasma MR-proADM levels have been observed in many clinical conditions associated with hypoxia and ischaemia, including chronic airway obstruction, peripheral arterial disease, ischaemic heart disease and CHF [13,14,15,16]. Population-based studies suggest that a high circulating level of MR-proADM is associated with increased risk of developing CHF [17, 18].
B-type natriuretic peptide (BNP), a peptide secreted primarily by the left ventricle when the heart is unable to efficiently pump blood, is released in equimolecular amounts with N-terminal pro-B-type natriuretic peptide (NT-proBNP) in response to increased parietal stress generated by increased ventricular fill pressures. With regard to the current European Society of Cardiology (ESC) recommendations, NT-proBNP is the gold standard biomarker for diagnosis of heart failure in cases of acute dyspnoea and for follow-up of people with chronic heart disease.
Studies on CHF prognosis have suggested the superiority of MR-proADM compared with NT-proBNP in terms of morbidity and mortality in individuals with post-acute coronary syndrome and CHF [19, 20]. In addition, in elderly individuals with acute dyspnoea, MR-proADM was found to be the only prognostic biomarker for cardiovascular mortality, compared with other biomarkers including BNP [21]. However, the primary outcome was not CHF but cardiovascular or all death. In addition, comparisons have focussed mainly on individuals in post-acute heart failure or on healthy populations and no prospective data are specifically available for individuals with type 2 diabetes. In the present investigation, we assessed and compared MR-proADM and NT-proBNP as predictors of CHF incidence in unselected individuals with type 2 diabetes.
Methods
Participants
We studied individuals with type 2 diabetes, from the Survival Diabetes and Genetics (SURDIAGENE) cohort, a hospital-based prospective monocentric study aiming to identify the genetic and environmental determinants of vascular complications in type 2 diabetes [22]. Individuals were recruited and followed regularly at the University Hospital of Poitiers, France, from 2002 to 2011. All participants gave written informed consent and study protocols were approved by the CCP Ouest III ethics committee of Poitiers.
Vital status and cardiovascular complications were determined from individuals’ hospital records and interviews with general practitioners and information was updated every second year. The latest update available (2015) was considered in the present analysis. Detailed description of study population, outcome criteria, adjudication procedure and biological procedures has been published previously [23]. Samples were stored at −80°C at the Poitiers biobanking facility (CRB 0033-00068; www.chu-poitiers.fr/specialites/centre-ressources-biologiques). Participants were considered regardless of whether or not they had a history of CHF at baseline. Individuals with end-stage renal disease (dialysis or renal transplant) at baseline were excluded from the current analysis.
Outcome
The primary endpoint of the study was the occurrence of CHF requiring hospitalisation during follow-up, as defined by the 2012 criteria of the ESC [24]. Endpoints were adjudicated by an ad hoc committee including diabetologists and cardiologists. According to the ESC 2012 Heart Failure guidelines, heart failure with reduced ejection fraction (HFrEF) was defined as heart failure with left ventricular ejection fraction (LVEF) <50% and heart failure with preserved ejection fraction (HFpEF) as heart failure with LVEF ≥50%. LVEF was determined by echocardiography performed by a cardiologist as part of standard care. We have previously shown an association between MR-proADM and all-cause death [16] and we searched for such a relationship with NT-proBNP.
Assays
MR-proADM concentration was measured in baseline plasma–EDTA samples by an automated immunofluorescent sandwich immune assay (B·R·A·H·M·S MR-proADM KRYPTOR; Thermo Fisher Scientific, Hennigsdorf, Germany) [12]. The limit of detection (LOD) was assessed as 0.05 nmol/l, intra-assay CV was 3.5–10% and the inter-assay CV was ≤20% for 0.2–0.5 nmol/l concentrations and ≤11% for 0.5–6 nmol/l concentrations.
NT-proBNP was measured in baseline plasma–EDTA samples by an electrochemiluminescence automated immunoassay (Roche Diagnostics, Mannheim, Germany). According to the manufacturer’s information, the LOD was 5 ng/l, the intra-assay CV was 1.2–1.9% and the inter-assay CV was 1.7–3.1%. Glomerular filtration rate was estimated by using the Chronic Kidney Disease Epidemiology (2009 CKD-EPI) creatinine equation [25].
Echocardiography
We considered a subgroup of individuals with clinically indicated echocardiography performed in routine care 12 months before or after inclusion (provided no CHF had occurred in the meantime), retaining those individuals without significant valvular disease (see electronic supplementary material [ESM] Fig. 1). Standard echocardiographic examinations were performed by trained cardiologists using a Vivid ultrasound device (GE-Vingmed Ultrasound, Horten, Norway) with a 2.5 MHz phased-array sector scan probe and second harmonic technology. Bidimensional, M mode and Doppler measurements were assessed following the American Society of Echocardiography (ASE) convention [26]. We considered five key variables: (1) left ventricular mass indexed for body surface area (M mode in parasternal long and short axis views, with the individual in the left lateral position); (2) LVEF calculated using biplane Simpson’s rule from the apical 4- and 2-chamber views; (3) left atrium area planimetered in apical 4-chamber view (dilatation >20 cm2); (4) Doppler-derived left ventricular diastolic inflow recorded in apical 4-chamber view, E and A peak velocities and their ratio E/A and (5) early diastolic transmitral peak velocities (E) to early peak diastolic velocities of the mitral annulus (Ea) and their ratio E/Ea.
Statistical analyses
Continuous variables are expressed as mean ± SD, or as median and interquartile range. Categorical variables are expressed as number of cases and percentage. Differences between groups were assessed by unpaired Student’s t test, ANOVA, Kruskal–Wallis test and Pearson’s χ2 test. Kaplan–Meier curves with a logrank test were used to plot and compare survival (CHF-free) rates over time by tertiles of plasma MR-proADM and NT-proBNP, respectively. Cox regression was used to examine the effect of explanatory variables on the hazard of CHF. HRs with their 95% CIs were computed in these analyses per SD increment. Variables presenting a significant univariate association with the outcome were all included in a multivariable Cox model. We censored individuals at time of primary endpoint or death or end of follow-up. As all-cause death could compete with the occurrence of our primary outcome, we performed competing-risk analysis according to the Fine and Gray method and presented subhazard ratios with their 95% CIs.
The integrated discrimination improvement (IDI) index was calculated to assess the improvement in 5 year risk prediction of MR-proADM and/or NT-proBNP in addition to traditional risk factors. A 5 year risk was selected because it approximates the median follow-up time for the study participants. We further analysed Harrel C-statistics according to the different models. The calculation of 95% CI of the difference between two models was performed with bootstrap and presented as mean ± standard error of the mean (SEM).
Statistical analyses were performed with SAS, version 9.3 (SAS, Cary, NC, USA). A p value <0.05 was considered significant.
A few participants did not have a full set of variables regarding NT-proBNP (n = 6). Participants with missing NT-proBNP values were removed from all the analyses including this covariate.
We conducted three complementary analyses. First, we assessed the impact of echocardiography data on the relationship between CHF and the studied biomarkers. Second, we assessed the improvement in Cox model performance by IDI accomplished by adding MR-proADM on top of covariates from the UKPDS Outcome Model for CHF [27], instead of the covariates derived from our model. Baseline covariates derived from the UKPDS Outcome Model study regarding hospitalisation for heart failure [27] include age, BMI, LDL-cholesterol, eGFR value <60 ml min−1 [1.73 m]−2, micro- or macroalbuminuria (vs normoalbuminuria), atrial fibrillation and a history of lower extremity amputation. Finally, stratification according to type of CHF was applied and data on HFpEF and HFrEF individuals were analysed separately.
Results
Out of the original 1468 SURDIAGENE participants, we analysed 1438 individuals (ESM Fig. 1): age 65 ± 11 years; 604 women and 834 men.
Plasma concentrations of MR-proADM and NT-proBNP and baseline characteristics
Plasma MR-proADM concentration was significantly higher in women than in men (0.76 ± 0.36 vs 0.72 ± 0.38 nmol/l, p = 0.004). The correlations of plasma MR-proADM with clinical and biological variables at baseline were previously reported [16]. Briefly, MR-proADM concentration was significantly and positively correlated with age, BMI and urinary albumin excretion and was negatively correlated with eGFR and HbA1c.
Plasma NT-proBNP concentration was significantly higher in women than in men, considering a post hoc analysis (348 [238–774] vs 258 [101–682] ng/l, respectively [p = 0.0178]) in the group with a history of CHD [n = 266 men and 118 women]), but not in the whole study population (106 [50–283] vs 120 [49–374] ng/l, respectively [p = 0.3260]). The increasing tertiles of NT-proBNP distribution were significantly and positively associated with age, systolic blood pressure, duration of diabetes, urinary albumin excretion and history of coronary heart disease and negatively associated with eGFR, HbA1c and BMI (ESM Table 1). We found a positive correlation between the concentrations of MR-proADM and NT-proBNP, both in men and women (r = 0.63 and r = 0.54, respectively, both p < 0.001).
Plasma concentration of MR-proADM and NT-proBNP and occurrence of CHF during follow-up
A total of 206 individuals required hospitalisation for CHF during follow-up (median 64 months). The incidence rate of the outcome was 2.5 (95% CI 2.2, 2.9) per 100 person-years. Characteristics of participants at baseline according to the occurrence of CHF during follow-up are shown in Table 1. Individuals with incident CHF, as compared with those without, were older, had a longer duration of diabetes, higher systolic blood pressure, lower eGFR, higher urinary albumin excretion and were more likely to have a previous history of coronary heart disease.
Incidence rates of CHF during follow-up by tertiles of baseline plasma MR-proADM and NT-proBNP concentrations are shown in ESM Table 2. Kaplan–Meier curves plotting CHF-free rates over time are shown in Figs 1 and 2. The higher tertile of plasma MR-proADM was associated with a significantly greater incidence of CHF (p < 0.0001). Similarly, the higher tertile of plasma NT-proBNP was associated with a greater incidence of CHF (p < 0.0001).
Baseline covariates entered in the regression models and results of multivariable analyses are shown in Table 2. Concentrations of plasma MR-proADM and plasma NT-proBNP remained significantly and independently associated with the incidence of CHF (adjHR [95% CI] 1.83 [1.51, 2.21] and 2.20 [1.86, 2.61], respectively, for a 1 SD increase in log10, both p < 0.0001). The inclusion of beta blocker use at baseline in the regression model had no impact on the association of the biomarkers with CHF (data not shown).
During follow-up, 417 participants died. Results were not modified when competing risk of all-cause death was accounted for and the subhazard ratio for plasma MR-proADM and plasma NT-proBNP remained significantly and independently associated with the incidence of CHF (adjsubHR [95% CI] 1.42 [1.17, 1.73], p = 0.0005 and 1.91 [1.61, 2.27], p < 0.0001, respectively, for a 1 SD increase in log10).
MR-proADM and NT-proBNP interaction as predictors of CHF
When MR-proADM was added to the multivariable Cox model including NT-proBNP, it was significantly associated with the incidence of CHF (adjHR [95% CI] 1.27 [1.03, 1.57], p = 0.03, for a 1 SD increase in log10 MR-proADM). However, MR-proADM did not significantly contribute supplementary information on prediction of 5 year risk of CHF in the model including NT-proBNP (IDI 0.0028 ± 0.003, p = 0.2664).
MR-proADM contributed significant supplementary information to the prognosis of heart failure when we considered the clinical risk factors (IDI [mean ± SEM] 0.021 ± 0.007, p = 0.0013).
When NT-proBNP was added to the multivariable Cox model including MR-proADM, it was significantly associated with the incidence of CHF (adjHR [95% CI] 1.98 [1.64, 2.40], p < 0.0001, for a 1 SD increase in log10 NT-proBNP). NT-proBNP significantly contributed complementary information on prediction of 5 year risk of CHF (IDI 0.028 ± 0.008, p = 0.0003) but not MR-proADM (Table 3). By contrast, MR-proADM did not contribute supplementary information on prediction of heart failure in a model including NT-proBNP (IDI [mean ± SEM] 0.003 ± 0.003, p = 0.2664), with similar results for heart failure with reduced ejection fraction and preserved ejection fraction.
The complementary information on prediction of 5 year risk of CHF generated by the inclusion of one biomarker or the other is summarised in Table 3.
Complementary analyses
Structural modification of the heart could be a key factor affecting the relationship between biomarkers and outcomes. As a complementary analysis, we present the HR of MR-proADM first, and NT-proBNP second, with adjustment on the echocardiography variables in the subgroup for which data were available (Table 4).
We repeated the multivariable Cox model using baseline covariates derived from the UKPDS Outcome Model study. When analysed separately, plasma MR-proADM and NT-proBNP were both associated with CHF (adjHR [95% CI] 1.77 [1.50, 2.10] and 2.27 [1.96, 2.64], respectively, for a 1 SD increase in log10, p < 0.0001 for both).
When considering MR-proADM and NT-proBNP in the same multivariable Cox model using baseline covariates derived from the UKPDS Outcome Model study, MR-proADM was not associated with CHF (adjHR [95% CI] 0.96 [0.77, 1.19], p = 0.69, for a 1 SD increase in log10 MR-proADM), while NT-proBNP remained associated with the outcome (adjHR [95% CI] 2.33 [1.91, 2.85], for a 1 SD increase in log10 NT-proBNP, p < 0.0001). NT-proBNP contributed significant supplementary information to the prediction model (IDI 0.0475 ± 0.0096, p < 0.001).
Among participants with incident CHF, 92 (44.7%) had HFrEF and 106 (51.4%) had HFpEF (ESM Table 3); ejection fraction status could not be ascertained for eight participants. MR-proADM and NT-proBNP were significantly and independently associated with incidence of HFrEF (ESM Table 4) and HFpEF (ESM Table 5).
In a multivariable Cox model analysis, when both biomarkers were accounted for simultaneously in the model, MR-proADM was not significantly associated with occurrence of HFrEF (p = 0.67) while NT-proBNP remained associated with the outcome (p < 0.0001). MR-proADM and NT-proBNP remained significantly associated with the occurrence of HFpEF (p = 0.0476 and p = 0.0009, respectively). When considered simultaneously in a prognostic model of HFpEF, neither NT-proBNP nor MR-proADM contributed significant supplementary information to the prediction model (IDI 0.0061 ± 0.0043 [p = 0.1559] and IDI 0.0037 ± 0.0028 [p = 0.17643], respectively).
Discussion
In the present investigation performed in a cohort of individuals with type 2 diabetes, plasma MR-proADM concentration was a predictor for the occurrence of CHF. The association was independent of other CHF risk factors identified in our cohort, such as a history of coronary heart disease, proteinuria and renal function. Moreover, MR-proADM contributed significant supplementary information to the prediction of CHF when we considered the clinical risk factors derived from both our analysis and the UKPDS Outcome Model [1]. Furthermore, NT-proBNP concentration was also a predictor for the occurrence of CHF independently of other CHF risk factors. MR-proADM did not contribute additional information to CHF when NT-proBNP was also included in the prediction model. Finally, NT-proBNP performs better than MR-proADM for the prognosis of CHF in people with type 2 diabetes, independently of HFrEF or HFpEF status.
The pathophysiological mechanisms linking ADM and heart failure make MR-proADM a very attractive prognostic biomarker for CHF. ADM is widely expressed, particularly in the heart, kidney and lung and in endothelial and smooth muscle cells of the vascular wall [28,29,30,31,32]. At physiological concentrations, ADM has many systemic and local effects including vasodilation and hypotension; it increases cardiac output, glomerular filtration rate, diuresis and fractional excretion of sodium [33, 34]. ADM exerts a direct inotropic effect in the heart and increases myocardial contractility. Moreover, ADM-induced arterial and venous vasodilatation is associated with a decrease in cardiac preload and after-load, and with a reduction in total peripheral resistance [33]. Intravenous administration of ADM in individuals with previous myocardial infarction and left ventricular dysfunction enhanced left ventricular contraction and improved left ventricular relaxation without increasing myocardial oxygen consumption [34]. Acute and chronic administration of ADM in animal models of heart failure confirmed the beneficial effect of ADM on cardiac function [34].
Our results in participants with type 2 diabetes support MR-proADM as a valuable biomarker for the prognosis of CHF, even after coronary heart disease is accounted for, as established in several prospective studies in unselected populations. A recent study in a Dutch community-based cohort reported an association between MR-proADM concentration and the occurrence of new-onset heart failure with reduced left ventricular ejection fraction [17]. This slightly differs from our results, where the prognostic effect for CHF was found in individuals with either preserved or reduced ejection fraction. The prognostic value of MR-proADM concentration was found only in a subset of participants with a high-baseline cardiovascular risk profile [17], of whom only 7% (116 participants) had diabetes, as indicated by use of glucose-lowering medications. A prospective study from Austria conducted in 781 participants with type 2 diabetes, with median follow-up of 15 months, reported an association between baseline MR-proADM and a composite endpoint of death, heart failure, myocardial infarction, unstable angina, cardiac rhythm and conduction disorders, peripheral and central arterial occlusive disease and transient ischaemic attack or stroke [35]. Unfortunately, published material did not permit specific extraction of data on the prognostic value of baseline MR-proADM concentration on CHF. Another study also demonstrated that MR-proADM concentrations were predictive for heart failure in people with coronary artery disease, including 489 participants (22%) with diabetes [36]. Finally, a study of 8444 healthy participants from the Finnish general population [37], including 403 participants with diabetes followed up for 14 years, also suggested a good predictive value of ADM for the occurrence of heart failure.
Altogether, we can conclude that the value of MR-proADM concentration for predicting the occurrence of CHF has been established, in participants with type 2 diabetes specifically, as in non-diabetes populations, in accordance with data from individuals with heart failure.
Our key research question was indeed to compare the prognostic value of MR-proADM and NT-proBNP in CHF occurrence. NT-proBNP levels were higher in women than in men in the subgroup of participants who had a history of coronary heart disease but surprisingly not in the whole study population. This might represent sample fluctuations, since inverse correlation with BMI was demonstrated, as consistently found in the literature. We found that NT-proBNP performed better than MR-proADM, which has been controversial in previous reports. A study conducted in 745 UK participants with non-ST-elevation myocardial infarction reported that both MR-proADM and NT-proBNP levels at discharge were predictors of the occurrence of subsequent heart failure [14], although data on their respective prognostic value for CHF are not available, to our knowledge. In people with heart failure, both NT-proBNP and MR-proADM were associated with hospitalisation or death during follow-up (median 55 months) [38]. Only the FINRISK study has examined the prognostic values of both MR-proADM and NT-proBNP for occurrence of CHF [37]. MR-proADM compared better than other natriuretic peptides for the occurrence of heart failure and, in contrast with our results, improved the risk reclassification for heart failure beyond NT-proBNP [37]. In the FINRISK study, only 5.1% of study participants were classified as diabetic. In addition, the incidence rate for CHF was rather low (0.43 for 100 person-years) compared with the incidence found in our population (2.50 for 100 person-years). Whether the results were influenced by the diabetes status and/or by the different incidence rate is hard to establish, and future studies will help to clarify this discrepancy.
We found that both biomarkers were of good prognostic value, even after adjustment for echocardiography variables as shown in complementary analyses. Our finding in favour of NT-proBNP supports a conservative approach, keeping this biomarker as a reference for occurrence of heart failure in individuals with type 2 diabetes. As no specific intervention is based on the presence of one biomarker or the other, we strongly recommend retaining NT-proBNP with regard to risk of CHF. Results of our complementary analysis, suggesting that NT-proBNP was prognostic for both HFpEF and HFrEF while MR-proADM was prognostic for HFpEF only, also favour keeping NT-proBNP as the biomarker of reference. Of note, our results assessing NT-proBNP in HFpEF and HFrEF were very consistent with findings of a recent large-scale study, which showed a significant prognostic effect of BNP for both types of heart failure, with a higher HR for HFrEF than for HFpEF [39].
The main strengths of our investigation are a long follow-up period and an adequate power to specifically analyse the occurrence of CHF as a primary endpoint. The main limitations of our study are that a clear history of CHF at baseline could not be established solidly by participants’ interview only. Cardiac ultrasound data at baseline was fragmentary and, as a result, stratification of the analyses by baseline LVEF, which might have pathophysiological implications, was not feasible in our study for all participants. A caveat must be considered regarding echocardiography as participants with data available were not selected for research purposes but for routine examinations; additionally, operators were trained cardiologists but did not follow any specific protocol and no core centre was used for storing imaging data. Of note, elevated MR-proADM or NT-proBNP most likely reflects structural heart disease and thereby represents opportunities for intervention. However, echocardiography is not as easily available in standard diabetology management as a simple blood test and elevated biomarkers could be of interest to specifically screen those participants for undergoing echocardiography.
In conclusion, people with type 2 diabetes have a high risk for the occurrence of heart failure, which is often diagnosed belatedly. Our study in a cohort of individuals with type 2 diabetes showed that plasma MR-proADM concentration is an independent predictor for the occurrence of CHF but gives no significant supplementary information for prediction beyond NT-proBNP. NT-proBNP determination was shown to have beneficial effects for early treatment of CHF in a biomarker-based interventional trial [40]. Our current data support the performance of future trials based on this biomarker, specifically in individuals with type 2 diabetes.
Data availability
Data are available on request from the authors.
Abbreviations
- ADM:
-
Adrenomedullin
- BNP:
-
B-type natriuretic peptide
- CHF:
-
Congestive heart failure
- ESC:
-
European Society of Cardiology
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFpEF:
-
Heart failure with preserved ejection fraction
- IDI:
-
Integrated discrimination improvement
- LOD:
-
Limit of detection
- LVEF:
-
Left ventricular ejection fraction
- MR-proADM:
-
Mid-region pro-adrenomedullin
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- SURDIAGENE:
-
Survival Diabetes and Genetics
References
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) (1998) UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:854–865
Bell DS (2003) Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26:2433–2441
Bonneux L, Barendregt JJ, Meeter K, Bonsel GJ, van der Maas PJ (1994) Estimating clinical morbidity due to ischemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health 84:20–28
Adams KF Jr, Fonarow GC, Emerman CL et al (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149:209–216
Carrasco-Sanchez FJ, Gomez-Huelgas R, Formiga F et al (2014) Association between type-2 diabetes mellitus and post-discharge outcomes in heart failure patients: findings from the RICA registry. Diabetes Res Clin Pract 104:410–419
Zannad F, Briancon S, Juilliere Y et al (1999) Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL study. Epidemiologie de lʼInsuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol 33:734–742
Packer M (2018) Heart failure: the most important preventable and treatable cardiovascular complication of type 2 diabetes. Diabetes Care 41:11–13
Van den Berge JC, Constantinescu AA, Booiten HJ et al (2018) Short term and long-term prognosis of patients with acute heart failure with and without diabetes: changes over the last decades. Diabetes Care 41:143–149
Minamino N, Kikumoto K, Isumi Y (2002) Regulation of adrenomedullin expression and release. Microsc Res Tech 57:28–39
Cormier-Regard S, Nguyen SV, Claycomb WC (1998) Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 273:17787–17792
Nakamura M, Yoshida H, Makita S, Arakawa N, Niinuma H, Hiramori K (1997) Potent and long-lasting vasodilatory effects of adrenomedullin in humans. Comparisons between normal subjects and patients with chronic heart failure. Circulation 95:1214–1221
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2005) Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 51:1823–1829
Adlbrecht C, Hulsmann M, Strunk G et al (2009) Prognostic value of plasma midregional pro-adrenomedullin and C-terminal-pro-endothelin-1 in chronic heart failure outpatients. Eur J Heart Fail 11:361–366
Dhillon OS, Khan SQ, Narayan HK et al (2010) Prognostic value of mid-regional pro-adrenomedullin levels taken on admission and discharge in non-ST-elevation myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) II study. J Am Coll Cardiol 56:125–133
Melander O, Newton-Cheh C, Almgren P et al (2009) Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA 302:49–57
Velho G, Ragot S, Mohammedi K et al (2015) Plasma adrenomedullin and allelic variation in the ADM gene and kidney disease in people with type 2 diabetes. Diabetes 64:3262–3272
Brouwers FP, van Gilst WH, Damman K et al (2014) Clinical risk stratification optimizes value of biomarkers to predict new-onset heart failure in a community-based cohort. Circ Heart Fail 7:723–731
Nishida H, Horio T, Suzuki Y et al (2008) Plasma adrenomedullin as an independent predictor of future cardiovascular events in high-risk patients: comparison with C-reactive protein and adiponectin. Peptides 29:599–605
Klip IT, Voors AA, Anker SD et al (2011) Prognostic value of mid-regional pro-adrenomedullin in patients with heart failure after an acute myocardial infarction. Heart 97:892–898
Wang J, Tan GJ, Han LN et al (2017) Novelbiomarkers for cardiovascularriskprediction. J Geriatr Cardiol 14:135–150
Bahrmann P, Christ M, Hofner B et al (2016) Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur Heart J Acute Cardiovascular Care 5:568–578
Hadjadj S, Fumeron F, Roussel R et al (2008) Prognostic value of the insertion/deletion polymorphism of the ACE gene in type 2 diabetic subjects: results from the non-insulin-dependent Diabetes, Hypertension, Microalbuminuria or Proteinuria, Cardiovascular Events, and Ramipril (DIABHYCAR), Diabete de type 2, Nephropathie et Genetique (DIAB2NEPHROGENE), and Survie, Diabete de type 2 et Genetique (SURDIAGENE) studies. Diabetes Care 31:1847–1852
Gellen B, Thorin-Trescases N, Sosner P et al (2016) ANGPTL2 is associated with an increased risk of cardiovascular events and death in diabetic patients. Diabetologia 59:2321–2330
McMurray JJ, Adamopoulos S, Anker SD et al (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 14:803–869
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Lang RM, Badano LP et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM (2013) UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 56:1925–1933
Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T (1994) Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett 338:6–10
Sugo S, Minamino N, Kangawa K et al (1994) Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun 201:1160–1166
Sugo S, Minamino N, Shoji H et al (1994) Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun 203:719–726
Kitamura K, Kangawa K, Eto T (2002) Adrenomedullin and PAMP: discovery, structures, and cardiovascular functions. Microsc Res Tech 57:3–13
Parkes DG, May CN (1997) Direct cardiac and vascular actions of adrenomedullin in conscious sheep. Br J Pharmacol 120:1179–1185
Nishikimi T, Yoshihara F, Mori Y, Kangawa K, Matsuoka H (2003) Cardioprotective effect of adrenomedullin in heart failure. Hypertens Res 26(Suppl):S121–S127
Nagaya N, Goto Y, Satoh T et al (2002) Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J Cardiovasc Pharmacol 39:754–760
Maier C, Clodi M, Neuhold S et al (2009) Endothelial markers may link kidney function to cardiovascular events in type 2 diabetes. Diabetes Care 32:1890–1895
Wild PS, Schnabel RB, Lubos E et al (2012) Midregional proadrenomedullin for prediction of cardiovascular events in coronary artery disease: results from the AtheroGene study. Clin Chem 58:226–236
Funke-Kaiser A, Havulinna AS, Zeller T et al (2014) Predictive value of midregional pro-adrenomedullin compared to natriuretic peptides for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Ann Med 46:155–162
Holmeger P, Morten S, Egstrup M et al (2015) The influence of diabetes mellitus on midregional proadrenomedullin concentrations and pronostic value in heart failure outpatients. J Card Fail 21:250–257
De Boer RA, Nayor M, deFilippi CR et al (2018) Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol 3:215–224
Ledwidge M, Gallagher J, Conlon C et al (2013) Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 310:66–74
Acknowledgements
Participants and their general practitioners are warmly thanked. The centres and personnel involved in SURDIAGENE recruitment and adjudications, and the members of the SURDIAGENE study group are shown in the ESM. J. Arsham (CHU Poitiers, Poitiers, France) edited the English of the text. V. Le Berre (CHU Poitiers, Poitiers, France) is acknowledged for secretarial assistance. Some of the data were presented as an abstract at the Heart Failure 2017 and the 4th World Congress on Acute Heart Failure meeting in 2017.
Funding
The SURDIAGENE study was supported by grants from the French Ministry of Health (PHRC-Poitiers 2004, PHRC Interregional 2008), Association Française des Diabétiques (Research Grant 2003), Groupement pour l’Etude des Maladies Métaboliques et Systémiques (GEMMS Poitiers, France). The present work was supported by grants from ‘Groupement des Maladies Métaboliques et Systémiques’ (GEMMS). Plasma MR-proADM measurement was performed by Thermo Fisher Scientific (Hennigsdorf, Germany), in anonymised tubes, by personnel blinded to characteristics and outcomes of participants. The analysis and interpretation of the data has been done without the participation of these organisations and companies.
Author information
Authors and Affiliations
Consortia
Contributions
MF and GV researched data and wrote the manuscript. EG performed statistical analyses, contributed to discussion and reviewed/edited the manuscript. FF, SR, PS, KM, BG, P-JS, J-MH, DM, GD, MR, MM and RR contributed to analysis and interpretation of data and drafting the article or revising it critically for important intellectual content. SH designed the study, researched data, and wrote the manuscript. SH is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Plasma MR-proADM measurement was performed by Thermo Fisher Scientific (Hennigsdorf, Germany), in anonymised tubes, by personnel blinded to characteristics and outcomes of participants. No other potential duality of interest relevant to this article was reported.
Additional information
The SURDIAGENE study group members are listed in the Electronic supplementary material (ESM).
Electronic supplementary material
ESM
(PDF 284 kb)
Rights and permissions
About this article
Cite this article
Fraty, M., Velho, G., Gand, E. et al. Prognostic value of plasma MR-proADM vs NT-proBNP for heart failure in people with type 2 diabetes: the SURDIAGENE prospective study. Diabetologia 61, 2643–2653 (2018). https://doi.org/10.1007/s00125-018-4727-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4727-7