Abstract
Aims/hypothesis
This study compared survival rates and causes of death after stroke in diabetic and non-diabetic patients in Sweden. We hypothesised that differences in cardiovascular risk factors, acute stroke management or socioeconomic status (SES) could explain the higher risk of death after stroke in diabetic patients.
Methods
The study included 155,806 first-ever stroke patients from the Swedish Stroke Register between 2001 and 2009. Individual patient information on SES was retrieved from Statistics Sweden. Survival was followed until 2010 (532,140 person-years) with a median follow-up time of 35 months. Multiple Cox regression was used to analyse survival adjusting for differences in background characteristics, in-hospital treatment, SES and year of stroke. Causes of death were analysed using cause-specific proportional hazard models.
Results
The risk of death after stroke increased in diabetic patients (HR 1.28, 95% CI 1.25, 1.31), and this risk was greater in younger patients and in women. Differences in background characteristics, cardiovascular risk factors, in-hospital treatment and SES did not explain the increased risk of death after stroke (HR 1.35, 95% CI 1.32, 1.37) after adjustments. Diabetic patients had an increased probability of dying from cerebrovascular disease and even higher probabilities of dying from other circulatory causes and all other causes except cancer.
Conclusions/interpretation
Differences in cardiovascular risk factors, acute stroke management and SES do not explain the lower survival after stroke in diabetic compared with non-diabetic patients. Diabetic patients are at higher risk of dying from cardiovascular causes and all other causes of death, other than cancer.
Similar content being viewed by others
Introduction
Diabetes is a strong risk factor for stroke [1] and is associated with an increased risk of death in the first four weeks after the stroke [2–4]. The impact of diabetes on survival after stroke is more pronounced among women and younger people, and the disparity between individuals with or without diabetes has not changed over the past 20 years [5].
In Sweden, diabetes is more common among individuals with low socioeconomic status (SES) and among immigrants [6]. In individuals with diabetes, low SES is associated with higher mortality [7]. In the general population, higher mortality from stroke is noted among lower socioeconomic groups [8, 9] and this is partly explained by differences in risk factors [9]. Low income and lack of education predicts higher case fatality after the acute phase of the stroke in the Swedish Stroke Register [10].
Only a few studies report the long-term (>2 year) survival after stroke in people with diabetes [11, 12]. We recently reported a greater impact of diabetes on long-term survival after stroke in patients younger than 65 years than those 65–74 years of age in the Northern Sweden MONICA study [5]. Differences in survival increased over longer follow-up periods in younger patients but were stable in older patients indicating different mechanisms and possibly also different causes of death.
Because low SES is associated with both increased risk of diabetes and increased risk of fatal outcome after a stroke, SES could possibly explain the increased stroke fatality in diabetic patients. The nationwide Swedish Stroke Register (Riks-Stroke) contains data on more than 150,000 first stroke events and provides an opportunity to discern the independent effect of diabetes on survival after shorter or longer time periods while taking multiple risk factors into account.
Our aim was to determine whether differences in SES and traditional cardiovascular risk factors, severity of stroke or hospital treatment could explain the higher case fatality in diabetic patients. We also aimed to determine whether causes of short- and long-term death after stroke differed between patients with or without diabetes.
Methods
This study included 156,591 patients with a first stroke (ischaemic stroke or intra-cerebral haemorrhage, ICD-10 [www.who.int/classifications/icd/en/]: I61, I63 and I64) registered in the Riks-Stroke database from 2001 to 2009. Riks-Stroke was established in 1994 to monitor and support the improvement of quality of stroke care in Sweden, and all hospitals admitting acute stroke patients participate [13]. In 2009, the register included 76 hospitals and covered approximately 91% of adult stroke patients in Sweden when compared with hospital discharge diagnoses and taking into account a 6% overdiagnosis of acute stroke in routine clinical practice [14]. Information from Riks-Stroke includes background patient characteristics, stroke diagnosis, acute hospital care, discharge status and prescribed secondary prevention at hospital discharge (for more information see www.riks-stroke.org accessed 27 May 2013). The diabetes diagnosis in Riks-Stroke is registered according to hospital records and includes both type 1 and type 2 diabetes mellitus.
Dates and causes of death as of 31 December 2010 were retrieved from the Swedish Cause of Death Register managed by the National Board of Health and Welfare. Underlying causes of death were grouped into cerebrovascular diseases (ICD-10: I60–69), other circulatory diseases (I00–I99, except for I60–69), cancer (C00–D48), diabetes (E10–E14) and other causes. If the underlying cause of death was reported to be diabetes and multiple causes were reported, the first registered multiple cause was used.
Individual patient information concerning education, income and country of birth were retrieved for the year preceding the stroke through linkage with the LISA database (longitudinal integration database for health insurance and labour market studies) managed by Statistics Sweden. Income was coded as high (the 10% highest incomes), low (the 10% lowest incomes) or middle income. Highest attained education was coded as primary school, secondary school or university. Information on education was missing for a large proportion of older patients (34.0% of patients ≥75 years) so the analysis that included education was restricted to patients younger than 75 years. The registers were linked using the Swedish personal identification number. The study was approved by the regional ethics review board.
Patient characteristics are presented as means and percentages separately for diabetic and non-diabetic stroke patients. Differences were compared with the t test for continuous variables and with the χ 2 test for categorical variables. Survival is presented by Kaplan–Meier curves, and differences in survival between diabetic and non-diabetic patients were compared with the logrank test. Different multiple Cox regression models were used to adjust for differences in background characteristics by adding in-hospital treatment and SES and stratifying for year of stroke. Preliminary analysis of overall survival, including age in 10-year intervals, showed an approximately linear effect of age, hence age was included as a linear effect in the model. Outcomes are presented as HRs with 95% CIs. To test if the reduced survival was more pronounced in some patient groups based on sex, age or SES, interactions were added to the model. Short-term survival (≤ 28 days) and long-term survival (patients surviving >28 days) were analysed in separate models.
To investigate the importance of the various diseases underlying the case fatality structure, cause-specific proportional hazard models were used. These clarify how different factors influence the immediate risk a patient has of dying from a specific disease at any point in time. From these models, the probability of dying from a given cause before a given time (the cumulative incidence) was calculated. The causes of death considered were cerebrovascular diseases, other circulatory diseases, cancer, and all other causes combined. The effects of diabetes on the various causes are given as HRs corrected for sex, year of stroke and age. For the HR for age, a flexible piecewise linear model with attached knots at every decade from 40 to 90 years of age was used, and this allowed for changing age effects in the very young and very old. All models used piecewise constant HRs with a breakpoint at day 28 to separate the effects of the acute phase from those of the long-term follow-up.
A p value of <0.05 was considered statistically significant. The survival package in R 2.15.0 (The R Foundation for Statistical Computing) was used to analyse cause of death. SAS 9.3 (SAS Institute, Cary, NC, USA) and IBM SPSS Statistics for Windows 21.0 (IBM, Armonk, NY, USA) were used for other statistical analysis.
Results
Of the 156,591 patients with a first stroke, 785 (0.5%) were missing information on diabetes. Thus, a total of 155,806 patients were included in the main analyses, of which 29,580 (19.0%) had diabetes, a frequency that remained at a similar level from 2001 to 2009. Patients were followed for a total of 532,140 patient-years, and the median follow-up times were 32 months for diabetic patients and 36 months for non-diabetic patients.
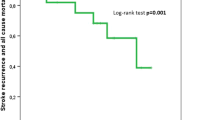
During follow-up, 15,805 (53.4%) of the diabetic patients and 58,107 (46.0%) of the non-diabetic patients died. The median survival after stroke was 55 months (95% CI 54, 56 months) in diabetic patients compared with 72 months (95% CI 71, 73 months) in non-diabetic patients. Survival after stroke was reduced in diabetic patients compared with non-diabetic patients among both women and men and in all age groups (p < 0.001, logrank test, Fig. 1). The observed difference in survival was small during the first month and increased with time (Fig. 1).
Compared with non-diabetic patients, diabetic patients were more often men, living in an institution, dependent in activities of daily living before stroke, had atrial fibrillation or had taken blood pressure lowering drugs before their stroke. Smoking and haemorrhagic stroke were less common among diabetic stroke patients (Table 1). In the age group 18–80 years with ischaemic stroke, diabetic patients were less likely to receive thrombolytic therapy. Secondary prevention has been recorded in the same way in Riks-Stroke since April 2004. In 94,207 patients who were discharged from hospital after that date, diabetic patients received similar or more intense secondary prevention than did non-diabetic patients (77.2% vs 62.5% were prescribed with an antihypertensive drug at hospital discharge, 81.7% vs 80.7% of ischaemic stroke patients received an antiplatelet drug, 52.0% vs 43.3% of ischaemic stroke patients received statins, and 34.3% vs 35.8% of ischaemic stroke patients with atrial fibrillation received warfarin). Diabetic stroke patients had lower income and lower education levels than non-diabetic patients and were more often born outside Sweden (Table 1).
The unadjusted HR comparing diabetic with non-diabetic patients was 1.10 (95% CI 1.06, 1.14) for short-term survival and 1.28 (95% CI 1.25, 1.31) for long-term survival. Differences in demographics, cardiovascular risk factors, in-hospital treatment, and SES could not explain the reduced survival (Table 2). Adding interactions to a regression model of the entire time interval showed that the difference in survival between diabetic and non-diabetic patients was more pronounced in younger patients (p < 0.001) and in women (p = 0.008) (Fig. 1). The association between lower education and reduced survival was similar in diabetic and non-diabetic patients.
Compared with non-diabetic patients, diabetic patients had a higher probability of dying from cardiovascular diseases and from other causes, other than cancer (Fig. 2). The impact of diabetes on the estimated probability of dying from cerebrovascular causes before 28 days was small, for example for a 75-year-old man admitted with acute stroke in 2009, the predicted probability was 6.5% in diabetic patients vs 5.7% in non-diabetic patients. For other circulatory causes, the corresponding probability was 2.3% vs 1.8%. For cancer death, the probability in diabetic patients was 0.6%, which was lower than the 0.7% in non-diabetic patients. The cause-specific HRs within the first 28 days were 1.15 (95% CI 1.10, 1.20) for cerebrovascular death, 1.32 (95% CI 1.23, 1.41) for other circulatory causes, 0.81 (95% CI 0.65, 1.01) for cancer and 1.32 (95% CI 1.13, 1.54) for other causes of death. The predicted probability for a 75-year-old man admitted with acute stroke in 2009 of dying from cerebrovascular causes within 5 years from stroke was 14.3% in diabetic patients vs 12.4% in non-diabetic patients. Corresponding probabilities were 15.2% vs 10.6% for other circulatory diseases, 7.3% vs 7.7% for cancer and 11.6% vs 8.0% for other causes of death, respectively. Over the entire follow-up, beyond 28 days, diabetes had a significant effect on cerebrovascular death (HR 1.25, 95% CI 1.20, 1.30) and a stronger effect on death from other circulatory causes (HR 1.59, 95% CI 1.54, 1.64) and on causes other than cancer (HR 1.60, 95% CI 1.53, 1.66). No significant effect was seen on death from cancer (HR 1.05, 95% CI 0.99, 1.12).
Evolution of the cause-specific cumulative short- and long-term incidences of cerebrovascular (light grey), other circulatory (grey), other causes of death except cancer (dark grey), and cancer (black) for a 75-year-old man admitted with acute stroke in 2009. Non-diabetic patients (a, c), diabetic patients (b, d), 28 days case fatality (a, b) and long-term survival (c, d)
Discussion
By analysing more than 150,000 Swedish patients suffering their first stroke over a period of 10 years, we have found that the risk of death during long-term follow-up is increased in diabetic stroke patients and that this risk is increased more in younger patients and in women. Differences in cardiovascular risk factors, acute stroke management and SES do not explain the lower survival. Over the entire 10 year follow-up period, the effect of diabetes has a limited, but significant, effect on cerebrovascular death and a stronger effect on other circulatory causes of death and on other causes except for cancer.
The strength of our study is that we include stroke patients from a nationwide register with high coverage. Owing to the large number of patients, long-term follow-up, and very low attrition rates, our estimates have high validity and representation. Data on possible confounders such as premorbid conditions, hospital treatment, stroke severity and discharge medication as well as income and immigration status were available for most patients. However, patients dying very early after admission are overrepresented among patients not included in Riks-Stroke [15]. This may have impacted on absolute percentages of survival during the 0–28 day interval but is unlikely to have affected the difference between diabetic and non-diabetic patients. The validity of recorded causes of death could be questioned, especially among elderly patients, but this should be non-differential regarding patients with or without diabetes thus not likely to introduce any major bias. The diabetes diagnosis was registered according to hospital records and has not been validated. Thus, we cannot exclude a potential dilution bias affecting the magnitude of the differences seen between diabetic and non-diabetic patients. In Riks-Stroke, the strategy has been to keep the registration simple to maintain a high patient coverage. We cannot rule out residual confounding due to unmeasured factors.
Our finding of increased case fatality among diabetic stroke patients corroborates two small Danish studies with follow-up periods of 10–12 years [11, 12] and two recent reports from the Northern Sweden MONICA study of long-term survival after a first stroke [5] or myocardial infarction [16]. Although limited to patients below the age of 75 years, a stronger impact of diabetes on stroke survival was noted among younger and female patients in the MONICA study. In this current study using the Riks-Stroke data, these results were extended to all ages and it was evident that at ages above 75 years the effect of diabetes decreases, which has not previously been described. A German study based on data from a health insurance company followed patients with a first stroke for up to five years and noted significantly lower case fatality among men with diabetes during the first month [17], which we found no evidence for.
Our results refute our original hypothesis that higher case fatality after stroke in diabetic patients is caused by lower SES [6, 8, 9] even though lower levels of education among stroke patients with diabetes was associated with worse prognosis. Other factors that could lead to lower survival, such as living in institutions, being dependent in activities of daily living, and having atrial fibrillations, were more common among diabetic patients while others were less common, such as being a smoker or having a haemorrhagic stroke. Thrombolysis was more often given to those stroke patients without diabetes, but in the MONICA study the gap in survival did not change when thrombolysis was introduced in Sweden [5] and adjustment for this treatment difference did not influence estimates of case fatality.
There is a paucity of data on causes of death after stroke and no studies on first stroke and diabetes. A long-term follow-up of the Danish MONICA study recorded cardiovascular causes of death in two thirds of patients who died within five years after stroke in the 1990s, and this result is similar to ours [18]. Early after stroke, cerebrovascular causes were more common in the Riks-Stroke registry but other circulatory diseases dominated later in the follow-up period. The higher fatality rate in diabetic patients was more pronounced for other circulatory causes than for cerebrovascular causes of death, which may be one important clue to diabetic patients’ increased case fatality.
The decreased survival after stroke in patients with diabetes is very similar to that after myocardial infarction [16]. As increased mortality is mostly attributable to heart disease, similar mechanisms probably apply after the initial event of both stroke and myocardial infarction. Less intensive or less effective secondary prevention among diabetics after the event could contribute although there are no data to confirm or refute such a hypothesis. It is also possible that diabetic stroke patients may benefit from higher doses of antiplatelet drugs or lower targets for blood pressure or cholesterol treatment. Finally, it is probable that the deleterious effects of diabetes are not only conveyed by the impact of traditional risk factors but also by specific mechanisms, such as the formation of advanced glycation end-products, for which no treatment is currently available [19].
Our study is the largest study on prognosis after first-ever stroke among diabetic patients and includes data from half a million patient-years and almost 30,000 patients with diabetes. We have found solid evidence for a lower survival over the long-term and our results suggest that younger patients and women are at particularly high risk in relative terms. Low SES among patients with diabetes does not explain the higher case fatality. Because cardiovascular causes of death were more common in those with diabetes, aggressive secondary prevention aimed at coronary risk factors seems warranted.
Abbreviations
- LISA:
-
Longitudinal integration database for health insurance and labour market studies
- Riks-Stroke:
-
the Swedish Stroke Register
- SES:
-
Socioeconomic status
References
O’Donnell MJ, Xavier D, Liu L et al (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376:112–123
Jia Q, Zhao X, Wang C et al (2011) Diabetes and poor outcomes within 6 months after acute ischemic stroke: The China National Stroke Registry. Stroke 42:2758–2762
Winell K, Paakkonen R, Pietila A, Reunanen A, Niemi M, Salomaa V (2011) Prognosis of ischaemic stroke is improving similarly in patients with type 2 diabetes as in nondiabetic patients in Finland. Int J Stroke 6:295–301
Rautio A, Eliasson M, Stegmayr B (2008) Favorable trends in the incidence and outcome in stroke in nondiabetic and diabetic subjects: findings from the Northern Sweden MONICA Stroke Registry in 1985 to 2003. Stroke 39:3137–3144
Eriksson M, Carlberg B, Eliasson M (2012) The disparity in long-term survival after a first stroke in patients with and without diabetes persists: the Northern Sweden MONICA study. Cerebrovasc Dis 34:153–160
Eliasson M, Bostrom G (2006) Major public health problems - diabetes. Scand J Public Health Suppl 67:59–68
Nilsson PM, Johansson SE, Sundquist J (1998) Low educational status is a risk factor for mortality among diabetic people. Diabet Med 15:213–219
Cox AM, McKevitt C, Rudd AG, Wolfe CD (2006) Socioeconomic status and stroke. Lancet Neurol 5:181–188
Kerr GD, Slavin H, Clark D, Coupar F, Langhorne P, Stott DJ (2010) Do vascular risk factors explain the association between socioeconomic status and stroke incidence: a meta-analysis. Cerebrovasc Dis 31:57–63
Lindmark A, Glader E-L, Asplund K, Norrving B, Eriksson M (2013) Socioeconomic disparities in stroke case fatality – Observations from Riks-Stroke, the Swedish stroke register. Int J Stroke Accepted
Andersen KK, Olsen TS (2011) One-month to 10-year survival in the Copenhagen stroke study: interactions between stroke severity and other prognostic indicators. J Stroke Cerebrovasc Dis 20:117–123
Ronning OM, Stavem K (2010) Predictors of mortality following acute stroke: a cohort study with 12 years of follow-up. J Stroke Cerebrovasc Dis 21:369–372
Asplund K, Hulter Asberg K, Appelros P et al (2011) The Riks-Stroke story: building a sustainable national register for quality assessment of stroke care. Int J Stroke 6:99–108
Koster M, Asplund K, Johansson A, Stegmayr B (2013) Refinement of swedish administrative registers to monitor stroke events on the national level. Neuroepidemiology 40:240–246
Appelros P, Hogeras N, Terent A (2003) Case ascertainment in stroke studies: the risk of selection bias. Acta Neurol Scand 107:145–149
Eliasson M, Jansson JH, Lundblad D, Naslund U (2011) The disparity between long-term survival in patients with and without diabetes following a first myocardial infarction did not change between 1989 and 2006: an analysis of 6,776 patients in the Northern Sweden MONICA Study. Diabetologia 54:2538–2543
Icks A, Claessen H, Morbach S, Glaeske G, Hoffmann F (2012) Time-dependent impact of diabetes on mortality in patients with stroke: survival up to 5 years in a health insurance population cohort in Germany. Diabetes Care 35:1868–1875
Bronnum-Hansen H, Davidsen M, Thorvaldsen P (2001) Long-term survival and causes of death after stroke. Stroke 32:2131–2136
Koulis C, de Haan JB, Allen TJ (2012) Novel pathways and therapies in experimental diabetic atherosclerosis. Expert Rev Cardiovasc Ther 10:323–335
Acknowledgements
We are thankful to Riks-Stroke and participating hospitals.
Funding
This study was supported by grants from the Swedish Council for Working Life and Social Research (grant no. 2011-0657) and the Swedish Research Council (2011-2395 and 2012-5934). Riks-Stroke is funded by the National Board of Health and Welfare and the Swedish Association of Local Authorities and Regions.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
MEr, KA, and MEl designed the study. MEr and BVR performed statistical analysis. MEr and MEl drafted the manuscript and KA and BVR revised the manuscript for important intellectual content. All authors approved the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eriksson, M., Asplund, K., Van Rompaye, B. et al. Differences in cardiovascular risk factors and socioeconomic status do not explain the increased risk of death after a first stroke in diabetic patients: results from the Swedish Stroke Register. Diabetologia 56, 2181–2186 (2013). https://doi.org/10.1007/s00125-013-2983-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2983-0