Abstract
Aims/hypothesis
Pyruvate carboxylase (PC) or pyruvate dehydrogenase (PDH) is required to transfer carbons from pyruvate into the Krebs cycle. PC activity is preserved in the islets of obese animals, but it is reduced in the islets of animal models of type 2 diabetes, suggesting that PC is important in beta cell adaptation to insulin resistance and that PC reduction may lead to beta cell failure.
Methods
To confirm the significance of PC, we first lowered activity using Pc (now known as Pcx) small interfering RNA (siRNA) in INS-1 cells and in dispersed rat islet cells. Second, we overexpressed PC in INS-1 cells, and third, we inhibited PDH by overexpressing the gene encoding pyruvate dehydrogenase kinase 4 (Pdk4) in INS-1 cells.
Results
Treatment of INS-1 cells or dispersed rat islet cells with Pc siRNA resulted in a significant reduction in insulin secretion in both cell types and reduced proliferation in INS-1 cells. This treatment also reduced the content of oxaloacetate, malate and ATP, as well as the NADPH:NADP+ ratio and activity of the pyruvate–malate shuttle. Overexpression of PC in INS-1 cells led to an elevation of insulin secretion and cell proliferation, whereas inhibition of PDH activity by overexpressing Pdk4 in INS-1 cells did not reduce insulin secretion.
Conclusions/interpretation
Our findings indicate that the PC pathway in beta cells might play a key role in pyruvate metabolism, insulin secretion and cell proliferation.
Similar content being viewed by others
Introduction
In addition to the firmly established role of glycolysis in regulation of glucose-stimulated insulin secretion (GSIS), the mitochondrial component of glucose metabolism has also been implicated in pancreatic beta cell function. There are two pathways by which pyruvate can enter the mitochondrial Krebs cycle, catalysed by pyruvate carboxylase (PC) [1, 2] and pyruvate dehydrogenase (PDH) [3, 4]. Pyruvate is converted to the Krebs cycle intermediate oxaloacetate by PC and to acetyl-CoA by PDH [5]. In most cells the PDH pathway predominates. However, pancreatic beta cells produce unusually high levels of PC [6]. As a result, the beta cell is unique in that approximately equal amounts of pyruvate enter into the Krebs cycle via PC and PDH [7]. A primary role of PC in tissues such as liver and kidney is to provide a substrate for gluconeogenesis. However, as pointed out by MacDonald [8], beta cells lack the essential gluconeogenic enzyme phosphoenolpyruvate carboxykinase, and therefore the high levels of PC must be required for a different function in beta cells. PC is essential for anaplerosis, which is needed to maintain the Krebs cycle. Krebs cycle intermediates are depleted by many processes, including lipogenesis and synthesis of certain amino acids [9, 10]. PC is able to restore the lost intermediates by producing oxaloacetate from pyruvate. Oxaloacetate can be converted to malate by malate dehydrogenase, then malate is released to the cytosol, where it is converted back to pyruvate by malic enzyme to form the pyruvate–malate shuttle (PMS) [1] or pyruvate cycling [11, 12]. This shuttle requires the activity of PC and in beta cells it is capable of producing more NADPH than the pentose–phosphate shuttle [8].
A number of investigators have provided evidence that mitochondrial metabolism is coupled to insulin secretion through PC rather than PDH. Inhibition of PC with phenylacetic acid prevents GSIS [13–16]. 13C nuclear magnetic resonance (NMR) isotopomer analysis [11, 12] has shown a close correlation between flux through PC and the capacity of glucose to stimulate secretion. Entry of pyruvate into the Krebs cycle via PDH does not appear to be important, since activation of PDH had only a minor effect on glucose metabolism and insulin release [17]. In addition, a recent report [18] found that a single nucleotide polymorphism in the PC gene of African Americans is significantly associated with the magnitude of the acute insulin response.
Type 2 diabetes is characterised by insulin resistance and beta cell failure [19]. Once insulin resistance occurs, pancreatic beta cells must secrete more insulin to maintain normal glucose levels. Increased insulin secretion requires beta cell adaptation, a process that includes both enhanced insulin secretion and increased beta cell proliferation [20]. In type 2 diabetes, the failure of beta cell adaptation coincides with reduced activities of PC [1, 2] and PDH [3, 4]. We have provided evidence that PC is more important to beta cell adaptation than PDH [13, 14, 21, 22]. During compensated obesity, islet PDH but not PC is reduced [13, 14], indicating that normal PDH activity is not required for beta cell adaptation. In addition, the PC inhibitor phenylacetic acid reduces the beta cell proliferation response in 60% pancreatectomised rats [14] and in Zucker fatty rats [13].
Current evidence for a key role of PC in beta cell function is extensive, but is limited to correlation or is dependent on the relatively non-specific PC inhibitor phenylacetic acid. Recently more evidence was provided to support the importance of PC in beta cells [23, 24]. In the present study, we performed specific genetic manipulation of PC activity (up- or downregulation) to produce corresponding changes in beta cell proliferation and insulin secretion; effect of PDH inhibition on insulin secretion was also tested.
Methods
Animals
The principles of animal laboratory care under the guidelines of both NIH and the University of Louisville’s Animal Care Committee were followed strictly. Male Sprague–Dawley rats (Taconic, Germantown, NY, USA) weighing 120 to 150 g were used for this research.
Cell culture
Rat insulinoma INS-1 cell line was provided by G. H. Hockerman (Department of Medicinal Chemistry and Molecular Pharmacology, Purdue University, West Lafayette, IN, USA) and the cells (passages 40 to 60) were cultured as described previously [25].
Pc small interfering RNA sequence design
Pc (now known as Pcx) small interfering RNA (siRNA) and control sequence were designed and synthesised by Invitrogen (Carlsbad, CA, USA) (Electronic supplementary material [ESM] Table 1).
Pc siRNA transfection
Pc siRNA transfection was carried out according to Stealth RNA transfection protocol (Invitrogen) and the cells were cultured for up to 72 h.
Overexpression of PC and the gene encoding pyruvate carboxylase kinase 4 in INS-1 cells
Human PC cDNA was provided by the laboratory of B. H. Robinson (Departments of Biochemistry and Paediatrics, University of Toronto, Toronto, Toronto, ON, Canada). Mouse Pdk4 cDNA was a gift from the laboratory of R. A. Harris (Departments of Biochemistry and Molecular Biology, Indiana University, Indianapolis, IN, USA). The PC or Pdk4 gene was cloned into EcoRI site of pCDNA3 (Invitrogen) driven by cytomegalovirus promoter. Transfection of these genes into INS-1 cells was carried out according to the manufacturer’s instructions for Lipofectamine 2000 (Invitrogen). Cell clones with stable PC or PDK4 overproduction were selected using G418. Control cells were treated with a vector that contained neither the PC nor the Pdk4 gene; all other treatments were the same as for PC- or PDK4-treated cells. The experiments were carried out on an isolated clone with the highest level of overexpression of PC or Pdk4.
Measurements of protein and DNA contents
Protein content was assayed by a commercial kit that used BSA as standard (BioRad, Hercules, CA, USA). Cell DNA content was determined using a quantification kit (PicoGreen dsDNA; Molecular Probes, Eugene, OR, USA).
PC activity assay
PC activity was measured according to the method of MacDonald et al. [1] using [14C]NaHCO3 as a substrate.
Mitochondria isolation and PC protein detection
Islet mitochondria isolation and detection of PC protein were measured using the method of MacDonald et al. [6] with modification [26].
Active PDH activity assay
Active PDH was measured as described previously [4, 27]. Cell homogenates were used for the PDH activity assay.
[3H]Thymidine incorporation
[3H]Thymidine incorporation assay was performed by a previously described method [28]. Radioactivity in the membranes was counted by a scintillation counter (Packard Instrument, Ramsey, MN, USA).
Islet isolation and separation of islets into single cells, with subsequent Pc siRNA transfection
The islets were isolated from 10-week-old male Sprague–Dawley rats by an adaptation of the Gotoh method [29]. Islets were digested with 0.25% (wt/vol.) trypsin for 5 min and incompletely digested islets were separated into single cells by passing through a 200 μl pipette tip. Dispersed islet cells were cultured for 1 h at 37°C in RPMI 1640 and treated with Pc siRNA for 48 h as described above.
Insulin secretion and insulin assay
Insulin secretion was performed by a previously described method [21]. Insulin secreted into KRB was measured using an ELISA insulin assay kit (Linco Research, St. Charles, MO, USA).
Glucose use and oxidation
Glucose use was measured by the method of Ashcroft [30]. Glucose oxidation was measured by the method of Hosokawa [31].
NADPH and NADP+ measurement
The cells or islets (∼40 μg protein) were homogenised in 50 μl of buffer containing 10 mmol/l Tris, pH 8.1, 1% (wt/vol.) NP-40, 0.5 mmol/l PMSF and 0.5% (wt/vol.) Triton X-100. After standing on ice for 20 min, the homogenate was centrifuged for 2 min at 12,000×g. The supernatant fraction was combined at room temperature for 30 min with 5 μl of 2 mol/l NaOH to destroy NADP+ (for NADPH assay) or 5 μl of 2 mol/l HCl to destroy NADPH (for NADP+ assay), after which 5 μl of 2 mol/l HCl or 5 μl of 2 mol/l NaOH was added to neutralise pH to ∼7. NADPH or NADP+ contents in the extract were measured by a modified method based on a cycling method described by Lowry and Passonneau [32]. Cycling reagent (100 μl) containing 100 mmol/l Tris, 80 mmol/l HCl, 9 U/ml of type II glutamate dehydrogenase, 5 mmol/l α-ketoglutaric acid, 2 mmol/l glucose 6-phosphate, 0.1 mmol/l ADP, 10 mmol/l ammonium acetate, 0.1% (wt/vol.) BSA and 6 U/ml glucose-6-phosphate dehydrogenase was added to each tube, and tubes were then incubated for 60 min at 38°C, followed by heating to 100°C for 10 min to stop the reaction. After brief centrifugation (500 g, 2 min), a 100 μl sample was transferred to a UV cuvette that contained 0.9 ml reaction buffer consisting of 0.006 U/ml 6-phosphogluconate dehydrogenase, 30 μmol/l NADP+, 0.1 mmol/l EDTA, 30 mmol/l ammonium acetate and 5 mmol/l MgCl2 and stood at room temperature for 30 min. The NADPH formed was measured by a fluorometer at 340 nm excitation and 420 nm emission. NADPH and NADP+ standards (0–10 nmol) were run in parallel through the entire procedure, including the extraction steps. The NADPH:NADP+ ratios were calculated from the NADPH and NADP+ contents measured in each assay. The blank was obtained from reaction buffer that contained islet extract, but did not contain glucose-6-phosphate dehydrogenase and glutamate dehydrogenase.
ATP, oxaloacetate and malate content measurement, and malate release from isolated mitochondria
Cell extract preparation and ATP content measurement were performed using a commercial kit (Molecular Probes). Oxaloacetate and malate contents were measured from cell perchloric acid extract by the methods of Parvin et al. [33]. The method for malate release from isolated mitochondria has been previously described [22].
Data presentation and statistical methods
All data are expressed as mean ± SEM. The listed n values represent the number(s) of individual experiments performed. Comparisons between two groups were performed by Student’s t test. Comparisons between multiple groups were performed by one- or two-way ANOVA (Tukey’s post hoc test). A value of p < 0.05 was considered significant.
Results
PC siRNA significantly decreased PC activity and protein concentration in treated INS-1 cells
We used siRNA to reduce PC protein and activity in INS-1 cells. In our preliminary tests, we compared the inhibitory effects of three pairs of siRNA on PC activity and GSIS (data not shown), selecting that with the strongest effects (ESM Table 1) for this study. As shown in Fig. 1a, siRNA significantly decreased PC activity (45% of control, p < 0.01) at 48 h after treatment. We chose 48 h of siRNA treatment because inhibition of PC activity in preliminary studies was greatest at 48 h (data not shown). A representative western blot (Fig. 1a) showed that content of the 130 kDa PC protein was reduced. Quantification of the data is shown under the protein bands. The values of relative optical density (normalised to actin) were significantly reduced in the Pc siRNA-treated group (p < 0.05).
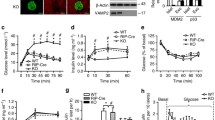
Pc siRNA decreases PC protein content, insulin secretion, cell proliferation and protein content in INS-1 cells treated for 48 h. (a) PC protein content and activity. The representative western blot (of a total of three) shows PC protein (∼130 kDa) and actin in control and Pc siRNA-treated cells. Protein band quantification (relative optical density) after normalisation to actin was 1.00 for control and 0.46 ± 0.08 (p < 0.05) for Pc siRNA. White circles, control; black circles, siRNA. (b) Glucose-stimulated (2.8, 5.5 and 16.7 mmol/l) insulin secretion. (c) KCl-stimulated (50 mmol/l) insulin secretion. (d) [3H]Thymidine incorporation. (f) Total protein content per well. Control, scrambled siRNA treatment. Data are mean±SEM, n = 4. *p < 0.05, **p < 0.01 vs controls
Pc siRNA significantly reduced GSIS in INS-1 cells
To test whether PC regulates insulin secretion, we inhibited PC activity by treating INS-1 cells with Pc siRNA for 48 h. The medium was then changed to Krebs–Ringer buffer and GSIS was performed in the presence of 2.8, 5.5 and 16.7 mmol/l glucose. Figure 1b shows that Pc siRNA significantly downregulated the glucose dose–response curve of insulin secretion in INS-1 cells. Pc siRNA, however, was unable to reduce KCl-induced insulin secretion (Fig. 1c), suggesting that the mechanism by which by KCl regulates insulin secretion is different from the PC pathway.
Pc siRNA significantly suppressed DNA and protein synthesis
We previously reported that changes in PC activity were associated with changes in beta cell proliferation [13, 14]. To test whether this was a causal relationship, we measured [3H]thymidine incorporation and protein content in Pc siRNA-treated INS-1 cells. Figure 1d demonstrates that [3H]thymidine incorporation in INS-1 cells treated for 48 h with Pc siRNA was decreased to 78% of control (p < 0.05). Parallel changes were seen in cell protein content in Pc siRNA-treated cells (Fig. 1e). Our data demonstrate that the specific inhibition of Pc expression by siRNA leads to a reduction in DNA and protein synthesis, which are measures of cell proliferation.
Inhibition of PC did not reduce glucose use and oxidation in INS-1 cells
We expected that inhibition of PC would lead to a reduction in glucose oxidation because about 50% of beta cell pyruvate enters the Krebs cycle by the PC pathway [7]. To test this, we measured glucose use and oxidation in INS-1 cells treated for 48 h with Pc siRNA. Surprisingly, glucose use and oxidation were not reduced (Fig. 2a, b). This result is consistent with that reported by Fransson et al. [16], who used phenylacetic acid (5 mmol/l) to inhibit PC in isolated rat islets; their results showed that phenylacetic acid was not able to inhibit glucose oxidation. We next measured PDH activity in these INS-1 cells. PDH activity was 2.7-fold increased (p < 0.01 vs control) (Fig. 2c). Interestingly, the increase in PDH did not compensate for the effect of reduced PC on GSIS and on beta cell proliferation (Fig. 1). The increase in PDH activity may be due to an increase in pyruvate concentration or a decrease in NADH:NAD+ ratio and may not be associated with a change in flux; this would explain the lack of change in glucose oxidation.
Pc siRNA significantly reduced GSIS in dispersed islet cells
Because INS-1 cells are a tumour cell line, we tested whether Pc siRNA regulates GSIS in primary beta cells. The islets isolated from male Sprague–Dawley rats were dispersed to single cells in order to improve the efficacy of siRNA treatment. Dispersed islet cells are less responsive to glucose [34], but dispersion is necessary for all cells to be exposed to the siRNA. As shown in Fig. 3a, we achieved a significant reduction in PC activity. Importantly, PC inhibition reduced GSIS significantly (Fig. 3b) when cells were stimulated with a high concentration of glucose (16.7 mmol/l). Figure 3b shows that glucose responsiveness in control group was poor. To find out the reasons for this, we tested the effect of transfection medium on primary beta cells and INS-1 cells. Our data show that GSIS in primary beta cells was significantly suppressed by the transfection medium, but INS-1 cells were less strongly affected (data not shown); this suggests chemical toxicity from the transfection medium and vulnerability in primary beta cells.
Pc siRNA treatment (48 h) in dispersed primary islet cells reduces PC activity (a) and leads to a reduction in glucose-stimulated (16.7 mmol/l) insulin secretion (b) and malate release from isolated mitochondria (mit.) (c). Control, scrambled siRNA. Data are the mean ± SEM, n = 4. *p < 0.5, **p < 0.01, ***p < 0.001; † p = 0.131
Intermediates and products of PMS and ATP content were reduced in beta cells treated with Pc siRNA
Evidence that the PMS is related to insulin secretion [11, 14] prompted us to measure oxaloacetate, malate contents and NADPH:NADP+ ratio, since they are the intermediates or products of this shuttle. As shown in Table 1, all of these variables were significantly reduced in Pc siRNA-treated INS-1 cells and in dispersed islet cells. NADPH:NADP+ ratios were significantly increased in response to high glucose (16.7 mmol/l) in control and siRNA-treated INS-1 cells and primary beta cells. This increase was inhibited by siRNA treatment or enhanced by PC overexpression. To test shuttle activity, we measured malate release from mitochondria isolated from Pc siRNA-treated dispersed islet cells and INS-1 cells. Malate release from Pc siRNA-treated INS-1 cells (Table 1) and from dispersed islet cells (Fig. 3c) was significantly reduced. These results confirm that PC controls flux through the PMS and that reductions in PC and shuttle activity coincide with reductions in insulin secretion and cell proliferation. Fransson et al. [16] have reported that PC is required for the fuel-induced rise in the ATP:ADP ratio. For this reason we measured ATP contents in Pc siRNA-treated INS-1 cells. As shown in Table 1, Pc siRNA significantly reduced ATP contents in these cells.
Elevation of PC significantly upregulated insulin secretion and cell proliferation in stable PC overexpressing INS-1 cells
Our results from Pc siRNA studies demonstrate that inhibition of PC reduced insulin secretion (Figs 1b, 3b) and cell proliferation (Fig. 1c, d). If elevation of PC leads to the opposite results, this would further support our hypothesis that PC has strong control in beta cell function. Therefore we overexpressed PC in INS-1 cells. The clone with the highest level of PC overexpression was obtained and used in present study. PC activity in this clone was increased about twofold (Fig. 4a); insulin secretion was increased 2.5-fold at 2.8 mmol/l glucose and threefold at 16.7 mmol/l glucose (Fig. 4b). Basal insulin secretion was also slightly but significantly increased in other clones overexpressing PC at lower levels (data not shown), suggesting that PC also controls basal insulin secretion. The stimulatory effect of PC overexpression on basal insulin secretion (2.8 mmol/l glucose) (Fig. 4b) is consistent with the inhibitory effect of Pc siRNA on basal insulin secretion (Fig. 1b). DNA and protein synthesis was significantly increased (Fig. 4c, d). Oxaloacetate and malate contents, NADPH:NADP+ ratio and malate release from mitochondria were also significantly elevated in PC overexpressing INS-1 cells (Table 1). All of these results demonstrate that PC plays a key role in insulin secretion and cell proliferation.
Stable overproduction (OVE) of PC in INS-1 cells upregulates insulin secretion and cell proliferation. (a) PC protein content and activity, respectively. Representative western blot shows PC protein (∼130 kDa) and actin (of a total of three). Protein band quantification (relative optical density) after normalisation to actin was 1.00 for control and 2.12 ± 0.12 (p < 0.05) for PC OVE. Glucose-stimulated (2.8 and 16.7 mmol/l) insulin secretion (b), protein content per well (c) and [3H]thymidine incorporation (d). Control cells were treated with the vector that did not contain PC gene. Data are mean ± SEM, n = 4. *p < 0.05, **p < 0.01 vs controls
Reduction of PDH by Pdk4 overexpression did not reduce insulin secretion
PDH activity was increased in Pc siRNA-treated INS-1 cells (Fig. 2c). Despite the increase in PDH activity, GSIS was reduced (Fig. 1b). This indicates that the PDH pathway may not be important for insulin secretion. To further test this, we developed INS-1 cells with reduced PDH activity. This was accomplished by taking advantage of the fact that PDH activity is inactivated by PDH kinase (PDK) through phosphorylation [35]. There are four PDK isoforms in pancreatic islets [27, 36]. We reduced PDH pathway by overproducing the PDK4 isoform in INS-1 cells. The clone with the highest level of Pdk4 overexpression was obtained and used in present study. Pdk4 overexpression in this clone produced a great reduction in PDH activity (Fig. 5a) but no significant change in PC activity (Fig. 5b). As shown in Fig. 5c, reduced PDH activity in INS-1 cells did not significantly change GSIS. Preliminary tests of glucose oxidation in INS-1 cells overexpressing Pdk4 indicated that [U-14C]glucose oxidation was not significantly changed (data not shown).
Reduction of active PDH activity by overproduction (OVE) of PDK4 (a) did not significantly change PC activity (b) or glucose-stimulated (2.8 and 16.7 mmol/l) insulin secretion (c) in INS-1 cells. Control cells were treated with the vector that did not contain the Pdk4 gene. Data are mean ± SEM, n = 4. **p < 0.01
Discussion
Glucose metabolism regulates insulin secretion [37–39] and blockade of glucose phosphorylation [40] or glycolysis [37] inhibits insulin secretion. Of the many enzymes and intermediates needed to complete glycolysis and glucose oxidation, it is uncertain which ones play a key role in the regulation of insulin secretion. Candidate enzymes include PC and PDH, which in pancreatic beta cells metabolise equal amounts of pyruvate into the Krebs cycle [7]. Previous studies [11, 12, 18], including those from our group [13, 14], suggested that PC might be important for insulin secretion and cell proliferation; however, none of those reports provided direct, causative evidence to confirm this hypothesis. In the current study, we performed the following specific genetic manipulations to test the role of PC vs PDH: (1) PC activity was decreased with siRNA in INS-1 cells and dispersed islet cells; (2) PC activity was increased by PC overexpression; and (3) PDH activity was reduced by Pdk4 overexpression in INS-1 cells. By gene manipulation, we demonstrated that reduction or elevation of PC activity caused corresponding changes in insulin secretion and cell proliferation. In contrast, PDH inhibition did not reduce insulin secretion. The inhibition of PC production by siRNA produced a remarkable elevation in PDH activity that could substitute for impaired PC activity in order to maintain normal rates of glucose oxidation. But elevated PDH could not compensate for the affect of diminished PC to reduce insulin secretion. This means that the essential role of PC does not stem from maintenance of glucose oxidation, but rather from its ability to feed substrate into anaplerosis and the PMS.
The part of the current study involving siRNA inhibition of the Pc gene was also recently carried out by Jensen et al. [23]. Parts of their results are consistent with our findings, while other results differ markedly. Thus, similarly to our data, Jensen et al. found that inhibition of PC (50% downregulation of PC) had no direct affect on either glucose use or oxidation. They also found that inhibition of PC reduced PMS, measured in our studies by malate release from mitochondria and in their studies via 13C NMR isotopomer analysis [23]. On the other hand, the two studies differed significantly in the effects of PC inhibition on beta cell content of NADPH or malate and on the effect of PC inhibition on insulin secretion. To explain the insulin secretion result, Jensen at al. performed radioisotopic and 13C-NMR-based measurements of metabolic flux and mass spectrometry-based metabolic profiling experiments; they found that an average 56% decrease in PC protein levels resulted in only about 20% reduction in flux through PC at stimulatory glucose and no significant difference in the increment of pyruvate cycling activity when glucose concentration was increased. They also found that acetyl-CoA and lactate levels are increased in INS-1 cells with decreased PC production, suggesting a compensatory mechanism in these cells. They also believed that PC inhibition can be compensated by pyruvate–isocitrate shuttle. These are the explanations of their insulin secretion data. In our study, however, inhibition of PC depleted both malate and NADPH. We had expected this since beta cell PC is a major source of malate and the malate–pyruvate shuttle is a major source of NADPH [8]. As we observed large reductions in NADPH:NADP+ ratios and Krebs cycle intermediates in siRNA-treated INS-1 cells (Table 1), it is possible that this contributes to the reduction in DNA synthesis and protein content (Fig. 1c, d). In contrast to our results, Jensen et al. [23] found that PC inhibition had no effect on either malate content or NADPH.
We are confident of our findings, since we not only found that PC inhibition depleted NADPH and malate content, but also that elevation of PC produced a corresponding increase in NADPH and malate contents. The other difference in the two studies is that we found insulin secretion to be sensitive to increased or decreased PC activity, while Jensen et al. [23] saw no impact of PC inhibition on GSIS. It is possible that the differences in results are due to differences in the INS-1 cell lines used by Jensen and by us. Different strains of INS-1 cells display very different characteristics for insulin secretion [41] and PMS [11]. For example, malate content at similar glucose concentrations was more than twice as high in Jensen’s cells than in our own. Also GSIS was many fold greater in Jensen’s cells. These differences could have been due to higher basal levels of PC activity in Jensen’s cells, which might be expected to reduce sensitivity to siRNA inhibition. However, the PC activity of Jensen’s cells is not known and the reason for the different findings remains uncertain. Very recently, this group [24] provided additional evidence to support an important role of the PMS in regulation of GSIS [21]. This is consistent with our conclusion.
The importance of the PC pathway for beta cell proliferation, a response essential to the beta cell’s adaptive response to insulin resistance, was demonstrated in the current study. Conversion of pyruvate to oxaloacetate, then to malate and back to pyruvate generates three important intermediates for cell proliferation: oxaloacetate, NADPH and malate. Oxaloacetate is used for protein and lipid synthesis [42–44], NADPH is needed for lipid and fatty acids synthesis [43, 44] and insulin secretion [8, 45], and malate is used for production of NADPH. Previous studies have suggested an important role for PC in beta cell proliferation. Thus we reported earlier that there is a more than a twofold increase in PC activity in islets from insulin-resistant, obese Zucker rats that have a 3.9-fold [13] increase in islet beta cell mass. Cline et al. [12] also reported that an approximately fourfold increase in anaplerosis, which provides substrate for proliferation, could be attributed to an increase in PC flux in INS-1 beta cells. We found that phenylacetic acid inhibition of PC prevents the full beta cell proliferative response to 60% pancreatectomy [14]. In addition, a proliferative role for PC is not unique to the beta cell. Mutations to PC in yeast cells inhibit growth [46, 47], and PC activity is elevated in growing tumour cell populations [48]. The current study provides the most specific and direct evidence of PC’s role in beta cell proliferation.
In summary, by manipulating PC and PDH levels, we have provided the most direct evidence that the PC pathway plays a key role in insulin secretion in dispersed islet cells and INS-1 cells, as well as in cell proliferation in INS-1 cells. Since the effects of PC were independent of total glucose metabolism and since the PDH pathway is much less important than PC, we suggest that it is the role of PC in anaplerosis and in the PMS pathway that makes it so important for insulin secretion.
Abbreviations
- GSIS:
-
glucose-stimulated insulin secretion
- NMR:
-
nuclear magnetic resonance
- PC:
-
pyruvate carboxylase
- PDH:
-
pyruvate dehydrogenase
- PDK:
-
pyruvate dehydrogenase kinase
- PMS:
-
pyruvate–malate shuttle
- siRNA:
-
small interfering RNA
References
Macdonald MJ, Tang J, Polonsky KS (1996) Low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of Zucker diabetic fatty rats. Diabetes 45:1626–1630
Macdonald MJ, Efendic S, Ostenson CG (1996) Normalization by insulin treatment of low mitochondrial glycerol phosphate dehydrogenase and pyruvate carboxylase in pancreatic islets of the GK rat. Diabetes 45:886–890
Zhou YP, Berggren PO, Grill V (1996) A fatty acid-induced decrease in pyruvate dehydrogenase activity is an important determinant of beta-cell dysfunction in the obese diabetic db/db mouse. Diabetes 45:580–586
Zhou YP, Ostenson CG, Ling ZC, Grill V (1995) Deficiency of pyruvate dehydrogenase activity in pancreatic islets of diabetic GK rats. Endocrinology 136:3546–3551
Wallace JC (1985) Pyruvate carboxylase. In: Keech DB, Wallace JC (eds) CRC series in enzyme biology. CRC Press, Boca Raton, pp 5–63
Macdonald MJ (1995) Influence of glucose on pyruvate carboxylase expression in pancreatic islets. Arch Biochem Biophys 319:128–132
Khan A, Ling ZC, Landau BR (1996) Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem 271:2539–2542
Macdonald MJ (1995) Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem 270:20051–20058
Jitrapakdee S, Gong Q, Macdonald MJ, Wallace JC (1998) Regulation of rat pyruvate carboxylase gene expression by alternate promoters during development, in genetically obese rats and in insulin- secreting cells. Multiple transcripts with 5′-end heterogeneity modulate translation. J Biol Chem 273:34422–34428
Jitrapakdee S, Wallace JC (1999) Structure, function and regulation of pyruvate carboxylase. Biochem J 340:1–16
Lu D, Mulder H, Zhao P et al (2002) 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci U S A 99:2708–2713
Cline GW, LePine RL, Papas KK, Kibbey RG, Shulman GI (2004) 13 C-NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem 279:44370–44375
Liu YQ, Jetton TL, Leahy JL (2002) Beta-cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem 277:39163–39168
Liu YQ, Han J, Epstein PN, Long YS (2005) Enhanced rat beta-cell proliferation in 60% pancreatectomized islets by increased glucose metabolic flux through pyruvate carboxylase pathway. Am J Physiol Endocrinol Metab 288:E471–E478
Farfari S, Schulz V, Corkey B, Prentki M (2000) Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49:718–726
Fransson U, Rosengren AH, Schuit FC, Renstrom E, Mulder H (2006) Anaplerosis via pyruvate carboxylase is required for the fuel-induced rise in the ATP:ADP ratio in rat pancreatic islets. Diabetologia 49:1578–1586
Nicholls LI, Ainscow EK, Rutter GA (2002) Glucose-stimulated insulin secretion does not require activation of pyruvate dehydrogenase: impact of adenovirus-mediated overexpression of PDH kinase and PDH phosphate phosphatase in pancreatic islets. Biochem Biophys Res Commun 291:1081–1088
Palmer ND, Langefeld CD, Campbell JK et al (2006) Genetic mapping of disposition index and acute insulin response loci on chromosome 11q: The Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 55:911–918
DeFronzo RA, Prato SD (1996) Insulin resistance and diabetes mellitus. J Diabetes Complicat 10:243–245
Leahy JL (1990) Natural history of beta-cell dysfunction in NIDDM. Diabetes Care 13:992–1010
Liu YQ, Moibi JA, Leahy JL (2004) Chronic high glucose lowers pyruvate dehydrogenase activity in islets through enhanced production of long chain acyl-CoA: prevention of impaired glucose oxidation by enhanced pyruvate recycling through the malate-pyruvate shuttle. J Biol Chem 279:7470–7475
Liu YQ, Tornheim K, Leahy JL (1999) Glucose-fatty acid cycle to inhibit glucose utilization and oxidation is not operative in fatty acid-cultured islets. Diabetes 48:1747–1753
Jensen MV, Joseph JW, Ilkayeva O et al (2006) Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J Biol Chem 281:22342–22351
Ronnebaum SM, Ilkayeva O, Burgess SC et al (2006) A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem 281:30593–30602
Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178
Han J, Xu J, Epstein PN, Liu YQ (2005) Long-term effect of maternal obesity on pancreatic beta cells of offspring: reduced beta cell adaptation to high glucose and high-fat diet challenges in adult female mouse offspring. Diabetologia 48:1810–1818
Xu J, Han J, Epstein PN, Liu YQ (2006) Regulation of PDK mRNA by high fatty acid and glucose in pancreatic islets. Biochem Biophys Res Commun 344:827–833
Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF (2004) Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes 53:149–159
Gotoh M, Maki T, Satomi S et al (1987) Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation 43:725–730
Ashcroft SJ, Weerasinghe LC, Bassett JM, Randle PJ (1972) The pentose cycle and insulin release in mouse pancreatic islets. Biochem J 126:525–532
Hosokawa H, Corkey BE, Leahy JL (1997) Beta-cell hypersensitivity to glucose following 24-h exposure of rat islets to fatty acids. Diabetologia 40:392–397
Lowry OH, Passonneau JV (1972) Enzymatic cycling. In: Lowry OH, Passonneau JV (eds) A flexible system of enzymatic analysis. Academic Press, New York, pp 129–145
Parvin R, Caramancion MN, Pande SV (1980) Convenient rapid determination of picomole amounts of oxaloacetate and aspartate. Anal Biochem 104:296–299
Ohgawara H, Kobayashi A, Chong SJ, Akaike T, Hashimoto Y (1994) Preparation of adult pig pancreatic cells: comparative study of methods with or without proteolytic enzymes. Cell Transplant 3:325–331
Krutzfeldt A, Spahr R, Mertens S, Siegmund B, Piper HM (1990) Metabolism of exogenous substrates by coronary endothelial cells in culture. J Mol Cell Cardiol 22:1393–1404
Sugden MC, Bulmer K, Augustine D, Holness MJ (2001) Selective modification of pyruvate dehydrogenase kinase isoform expression in rat pancreatic islets elicited by starvation and activation of peroxisome proliferator-activated receptor-alpha: implications for glucose-stimulated insulin secretion. Diabetes 50:2729–2736
Mertz RJ, Worley JF, Spencer B, Johnson JH, Dukes ID (1996) Activation of stimulus-secretion coupling in pancreatic beta-cells by specific products of glucose metabolism. Evidence for privileged signaling by glycolysis. J Biol Chem 271:4838–4845
Dukes ID, McIntyre MS, Mertz RJ et al (1994) Dependence on NADH produced during glycolysis for beta-cell glucose signaling. J Biol Chem 269:10979–10982
German MS (1993) Glucose sensing in pancreatic islet beta cells: the key role of glucokinase and the glycolytic intermediates. Proc Natl Acad Sci U S A 90:1781–1785
Matschinsky FM (2002) Regulation of pancreatic beta-cell glucokinase: from basics to therapeutics. Diabetes 51(Suppl 3):S394–S404
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49:424–430
Menendez J, Delgado J, Gancedo C (1998) Isolation of the Pichia pastoris PYC1 gene encoding pyruvate carboxylase and identification of a suppressor of the pyc phenotype. Yeast 14:647–654
Infante JP, Huszagh VA (1998) Analysis of the putative role of 24-carbon polyunsaturated fatty acids in the biosynthesis of docosapentaenoic (22:5n−6) and docosahexaenoic (22:6n−3) acids. FEBS Lett 431:1–6
Dmitriev LF (2001) Activity of key enzymes in microsomal and mitochondrial membranes depends on the redox reactions involving lipid radicals. Membr Cell Biol 14:649–662
Macdonald MJ (2003) Export of metabolites from pancreatic islet mitochondria as a means to study anaplerosis in insulin secretion. Metabolism 52:993–998
Stucka R, Dequin S, Salmon JM, Gancedo C (1991) DNA sequences in chromosomes II and VII code for pyruvate carboxylase isoenzymes in Saccharomyces cerevisiae: analysis of pyruvate carboxylase-deficient strains. Mol Gen Genet 229:307–315
Blazquez MA, Gamo FJ, Gancedo C (1995) A mutation affecting carbon catabolite repression suppresses growth defects in pyruvate carboxylase mutants from Saccharomyces cerevisiae. FEBS Lett 377:197–200
Liu KJ, Kleps R, Henderson T, Nyhus L (1991) 13C NMR study of hepatic pyruvate carboxylase activity in tumor rats. Biochem Biophys Res Commun 179:366–371
Acknowledgements
The preliminary data were presented at the 66th American Diabetes Association Scientific Sessions Annual Meeting, 2006, Washington, DC, USA. We thank G. H. Hockerman for providing INS-1 cell line, M. A. Carbone (Departments of Biochemistry and Paediatrics, University of Toronto, Toronto, Toronto, ON, Canada) for human PC cDNA and N. H. Jeoung (Departments of Biochemistry and Molecular Biology, Indiana University, Indianapolis, IN, USA) for mouse Pdk4 cDNA. This work was supported by grants to Y. Q. Liu from the National Institutes of Health (P20 RR/DE17702 from the COBRE Program of the National Center for Research Resources and 1R01 DK077624-01), the American Diabetes Association (Junior Faculty Award) and by grant HL075080 to P. N. Epstein. This project was also supported by a grant (6931) from The Research Institute for Children, Children’s Hospital at New Orleans.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Xu and J. Han contributed equally to this study.
Electronic supplementary material
Below is the link to electronic supplementary material.
ESM Table 1
The sequences designed for inhibiting rat Pc expression in INS-1 cells and dispersed rat islet cells (PDF 59.8 KB)
Rights and permissions
About this article
Cite this article
Xu, J., Han, J., Long, Y.S. et al. The role of pyruvate carboxylase in insulin secretion and proliferation in rat pancreatic beta cells. Diabetologia 51, 2022–2030 (2008). https://doi.org/10.1007/s00125-008-1130-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-1130-9