Abstract
Aims/hypothesis
Post-prandial glucose may be a risk factor for cardiovascular disease and chronic diabetic complications. We tested the hypothesis that post-prandial hyperglycaemia is common in type 2 diabetes, even among patients in apparently good glycaemic control, and that simple clinical characteristics identify subsets of diabetic patients with frequent post-prandial hyperglycaemia.
Subjects and methods
Three self-assessed daily blood glucose profiles over a 1-week period, including 18 glucose readings before and 2 h after meals, were obtained from 3,284 unselected outpatients (men 51%; age 63±10 years) with non-insulin-treated type 2 diabetes mellitus attending 500 different diabetes clinics operating throughout Italy.
Results
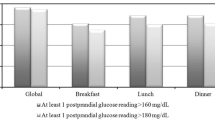
A post-prandial blood glucose value >8.89 mmol/l (160 mg/dl) was recorded at least once in 84% of patients, and 81% of patients had at least one Δglucose ≥2.22 mmol/l (40 mg/dl). Among patients with apparently good metabolic control, 38% had >40% of post-prandial blood glucose readings >8.89 mmol/l (≥4 of 9 meals in total), and 36% had >40% Δglucose ≥2.22 mmol/l. In multivariate analysis adjusted for pre-prandial glucose levels, older age, longer duration of diabetes, absence of obesity, hyperlipidaemia and hypertension, as well as treatment with sulfonylureas, were significantly associated with greater glucose excursions after meals.
Conclusions/interpretation
These results indicate that post-prandial hyperglycaemia is a very frequent phenomenon in patients with type 2 diabetes mellitus on active treatment; can occur even when metabolic control is apparently good; and can be predicted by simple clinical features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few years the concept has emerged that poorly controlled post-prandial glucose significantly contributes to high HbA1c concentrations and to the development of chronic diabetic complications, particularly cardiovascular disease (CVD) [1–4]. The adverse impact of post-prandial or post-challenge (OGTT) hyperglycaemia seems to be even stronger than that of fasting hyperglycaemia [5–9]. Nevertheless, the current consensus of the American Diabetes Association (ADA) is that post-prandial glucose should not necessarily be systematically monitored, and that post-prandial glucose peaks should not necessarily be targeted and corrected [10], although, if monitored, the recommended goal for post-prandial glucose is <10.0 mmol/l (180 mg/dl) [11]. Conversely, the guidelines of the Diabetes European Policy Group, under the auspices of the International Diabetes Federation (IDF), have recommended monitoring and controlling post-prandial hyperglycaemia, with a goal of <8.89 mmo/l (160 mg/dl) [12].
As far as we know, only in two small series of subjects with type 2 diabetes have data on circadian glucose levels been collected, and the results were partially conflicting [13–16]. As a consequence, our knowledge of the frequency and distribution of this metabolic abnormality is poorly defined. We conducted the present study in a very large sample of type 2 diabetic patients to test the hypothesis that post-prandial hyperglycaemia is common, even among patients apparently well-controlled by HbA1c, and to identify clinical predictors of unacceptably elevated post-prandial glycaemia.
Subjects and methods
Subjects
Consecutive patients with type 2 diabetes were recruited among outpatients regularly attending 500 Diabetes Clinics operating throughout Italy. Each clinic recruited at least ten patients. Exclusion criteria were as follows: (1) age <40 years; (2) current insulin treatment (because insulin absorption is variable and insulin dose is frequently changed by patients); (3) changes in treatment with oral antidiabetic agents in the last 3 months; (4) acute illness in the last 3 months; and (5) major comorbidity (e.g. malignancies, heat failure, liver or kidney failure). Information on diet and physical activity were collected by a structured interview and they had to be stable in the 3 months prior to the recruitment and throughout the study period. As for the standard guidelines, patients were on a diet providing about 55% of energy from carbohydrates, about 30% from lipids and about 15% from proteins. In general, the distribution of energy in the three main meals was ∼25% breakfast, ∼37.5% lunch and ∼37.5% dinner, and energy supply aimed at reducing body weight if excessive, or at maintaining body weight if satisfactory. All patients had been instructed on diet and on the glycaemic index of foods by a dietitian at each Diabetes Clinic at least 3 months prior to study. The diet was recommended to be followed every day of the study including those during which patients self-assessed blood glucose. Of course, the quality of food could be different in the various regions of Italy, but the structure of diet in terms of composition, number of meals and energy supply was very similar. There was no reason to think that adherence to diet and physical activity was different across centres. Furthermore, the very large number of subjects is expected to minimise possible variations. On the other hand, the great number of centres from all the regions produced a very representative sample.
Informed consent was obtained from every participant and the study was approved by the local ethics committee. Patients (who were not paid volunteers) had to be willing to perform home blood glucose monitoring and to be reliable in making this self-assessment. A total of 6,009 patients agreed to participate in the study and provided at least one home blood glucose assessment. Many of them provided several glucose readings. However, the present report focuses on the 3,284 subjects who provided complete home blood glucose assessments. Subjects providing complete glucose profiles had similar pre- and post-prandial glucose levels, and glucose changes after meals compared with those who provided incomplete data. We focused on subjects with complete data in order to have all statistical analyses carried out on the same sample of subjects.
Clinical and laboratory data
On the day of recruitment patients came to the clinics at 07.00–08.30 h after an overnight fast and had a venous blood sample taken from an antecubital vein for the laboratory assessment of fasting plasma glucose, plasma lipids (total and HDL-cholesterol and triglycerides) and HbA1c. Venous blood was centrifuged within 1 h after sampling and plasma used for the immediate assessment of glucose concentration (glucose oxidase method), plasma lipids (standard spectrophotometric methods) and HbA1c (HPLC; reference range in non-diabetic subjects 3.5–5.5%; DCCT standardised). Laboratory assessment was not centralised. However, all laboratories participated in quality control procedures for HbA1c and lipid assays. Dyslipidaemia was diagnosed when triglycerides were >1.70 mmol/l (150 mg/dl) and/or HDL-cholesterol was <1.28 mmol/l (50 mg/dl) in women or 1.02 mmol/l (40 mg/dl) in men and/or lipid-lowering drugs were used. Weight, height and blood pressure were assessed with standard clinical techniques. Overweight was defined by a BMI in the range 25–29.9 kg/m2, and obesity as a BMI ≥30 kg/m2. Blood pressure was measured with a mercury manometer, on the right arm, after 5 min of rest in the sitting position. Hypertension was diagnosed when systolic blood pressure was ≥130 mmHg and/or diastolic blood pressure was ≥85 mmHg and/or antihypertensive medications were used.
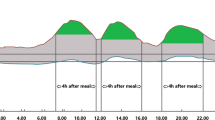
All subjects were given the same model of glucose meter (Euroflash; Life Scan, Milpitas, CA, USA) and were instructed about its use. They were then asked to measure blood glucose at home just before and about 2 h after the beginning of breakfast, lunch and dinner on 3 days over the period of 1 week, after a period of 1–2 weeks of familiarisation with glucose self-assessment with such a device.
Assessment of glucose control
Overall glucose control was considered satisfactory when HbA1c was <7% [11, 12]. Following the guidelines for diabetes care of the European Diabetes Policy Group [12], an absolute post-prandial blood glucose level >8.89 mmol/l (160 mg/dl) was considered unsatisfactory. These guidelines are consistent with the current ADA recommendations as a glucose concentration of 8.89 mmol/l in whole blood approximates 10 mmol/l in the plasma. For the purposes of this analysis we defined an absolute increase in blood glucose level following the meal of ≥2.22 mmol/l (40 mg/dl), and a relative increase ≥25% as exaggerated [13]. A value of ≥2.22 mmol/l corresponds to the difference between the upper limit of blood glucose targets in the pre-prandial state (6.66 mmol/l) and in the post-prandial state (8.89 mmol/l), and is the magnitude of post-challenge glycaemic excursions independently associated with increased risk of CVD events [17].
Statistical analysis
Standard procedures were used to calculate means, SDs, medians and linear correlation coefficients. We used mixed-effects ANOVA models accounting for repeated measures within subjects to predict levels of post-prandial glucose, Δglucose or Δ% glucose, or proportions with post-prandial glucose >8.89 mmol/l, Δglucose ≥2.22 mmol/l or Δ% glucose ≥25. Variables predicting post-prandial glucose levels were pre-prandial glucose (≤6.66, 6.67–8.89, 8.90–11.1, >11.1 mmol/l, corresponding to ≤120, 121–160, 161–200, >200 mg/dl), sex, age (≤50, 51–60, 61–70, >70 years), time since diagnosis (≤3, 4–6, 7–12, >12 years), BMI (normal, overweight, obesity), hypertension (no, yes), dyslipidaemia (no, yes) and diabetes treatment (sulfonylureas alone, metformin alone, both drugs, other combination, only diet). We used SPSS software, and considered a p value <0.05 to be statistically significant.
Results
Study subjects providing complete glucose profiles had similar demographic and clinical features compared with subjects not providing complete glucose profiles (Table 1). Among subjects providing complete glucose profiles, those with HbA1c <7 and ≥7% had quite similar clinical features. Only time since diagnosis, smoking, fasting plasma glucose and treatment were different.
Mean blood glucose levels before and following the three main meals and mean changes (absolute, relative) in blood glucose after meals among 3,284 patients providing complete profiles are reported in Table 2. Pre-meal blood glucose levels were, on average, quite similar and the Δglucose and Δ% glucose increases with lunch and dinner were virtually identical. A lower increase in blood glucose was observed following breakfast than following lunch or dinner. Higher levels of blood glucose and higher Δglucose following the meals were observed in patients with HbA1c ≥7% compared with those with HbA1c <7%. However, the Δ% glucose following lunch and dinner were slightly lower in subjects with poorer control than in those with better control. Subjects with poorer control had average pre-prandial, post-prandial and Δglucose higher than subjects with better control. However, the former and the latter had no significantly different average Δ% glucose following meals.
When we examined how frequently high glucose peaks and exaggerated glucose excursions with meals occurred, we found that both events were very common in subjects with both better control (HbA1c <7%) and poorer control (HbA1c ≥7%) (Table 3). Among subjects with apparently good control, only 28.3% never recorded a post-prandial glucose >8.89 mmol/l, only 22.3% never had a Δglucose ≥2.22 mmol/l and only 11.5% never had a relative increase of blood glucose ≥25% after meal. We also assessed rates of hypoglycaemia (defined as a glucose level <3.88 mmol/l [70 mg/dl] on any of the six daily glucose assessments) according to the degree of diabetes control. Absolute rates of hypoglycaemia were very low, but were statistically higher (p<0.0001) in patients with HbA1c <7% (0.88% of 23,094 glucose tests [1,283 patients×18 tests]) compared with patients with HbA1c ≥7% (0.50% of 36,018 glucose tests).
High correlations were found between pre- and post-prandial glucose levels, with r values about 0.60–0.70. Accordingly, when subjects were grouped according to mean pre-prandial glucose, we found that mean post-prandial glucose increased across groups. However, absolute and relative changes in blood glucose after a meal were negatively correlated with pre-prandial glucose levels, with r values in the range 0.20–0.50. All these negative correlations were highly significant (p<0.001). Accordingly, Δglucose and, to a greater extent, Δ% glucose significantly decreased across groups of pre-prandial glucose levels (Table 4). Among subjects with a satisfactory mean pre-prandial glucose (≤6.66 mmol/l; n=1,057), 32% had a mean Δglucose ≥2.22 mmol/l and 59% had a mean Δ% glucose >25. On the other hand, among subjects with a very poor mean pre-prandial glucose (>11.1 mmol/l; n=163), 67% had a mean Δglucose <2.22 mmol/l, and 80% had a mean Δ% glucose <25.
In the whole sample, pre-prandial and, to a lesser extent, post-prandial glucose levels were significantly correlated to HbA1c. In multivariate analysis, pre-prandial glucose was more strongly associated with HbA1c than post-prandial glucose. We also assessed the relative contribution of pre- and post-prandial glucose to HbA1c concentrations with multivariate analysis after stratifying subjects into three categories according to HbA1c levels (<6.50, 6.50–7.49, ≥7.5%) and by modelling HbA1c as a function of pre-prandial glucose (per mmol/l increase, mean of nine readings) and post-prandial glucose (mean of nine readings). In subjects with very good control (HbA1c <6.5%), post-prandial (p=0.006) but not pre-prandial glucose (p=0.06) was associated with HbA1c, whereas pre-prandial (p<0.0001) but not post-prandial (p=0.5) glucose was significantly associated with HbA1c in subjects with poor control (HbA1c ≥7.5%). In subjects with HbA1c 6.5–7.49%, pre- and post-prandial glucose were associated with HbA1c to a similar extent (both p≤0.03).
We examined the frequency of the different glycaemic phenotypes. Subjects were categorised in six groups according to a good, fair or poor mean glucose level in the pre-prandial state (≤6.66, 6.67–8.89, ≥8.90 mmol/l) and a satisfactory or exaggerated mean absolute glucose excursion after meals (<2.22 or ≥2.22 mmol/l). The most common phenotype was fair pre-prandial with satisfactory post-prandial excursion (n=1,120, 34%), followed by good pre-prandial and satisfactory post-prandial excursion (n=718, 22%), and the least common phenotype was poor pre-prandial and exaggerated post-prandial (n=203, 6%). Among subjects with poor pre-prandial glucose levels (n=684), those with satisfactory post-prandial excursions were twofold more common than those with exaggerated post-prandial excursions (481 vs 203). Among subjects with good pre-prandial glucose levels (n=1,062), about one-third (n=344) had exaggerated post-prandial glucose excursions after meal. The same held true for subjects with fair pre-prandial glucose levels (n=1,538) of whom about one-third (n=418) had exaggerated glucose excursions with meals.
Mean post-prandial glucose but not Δglucose or Δ% glucose was significantly higher in women than in men (9.4±2.22 vs 9.3±2.11 mmol/l, p=0.01). Age was positively correlated with mean post-prandial glucose (r=0.089, p<0.0001), Δglucose (r=0.064, p=0.0003) and Δ% glucose (r=0.048, p=0.005). Duration of diabetes was positively correlated with average post-prandial glucose (r=0.190, p<0.0001), Δglucose (r=0.108, p<0.0001) and Δ% glucose (r=0.064, p=0.0003). BMI was negatively correlated with mean Δglucose (r=−0.073, p<0.0001) and Δ% glucose (r=−0.098, p<0.0001).
Subjects were also grouped according to diabetes treatment (diet only, sulfonylureas alone, metformin alone, both drugs, other combinations). We observed that mean post-prandial glucose, Δglucose and Δ% glucose were significantly different in these groups. In particular, after adjusting for sex, age, duration of diabetes and pre-prandial glucose, subjects treated with sulfonylureas alone or in combination with biguanide had mean post-prandial glucose (p<0.0001), average Δglucose (p=0.0005) and mean Δ% glucose (p=0.008) higher than those observed in subjects treated with diet only or with metformin alone.
Simple clinical characteristics may be useful to identify subsets of patients likely to have exaggerated post-meal glucose response. In mixed-effects ANOVA models adjusted for pre-prandial glucose level, older age, longer duration of diabetes, normal body weight, absence of hypertension, absence of dyslipidaemia, and treatment with sulfonylureas alone or in combination were independently associated with higher post-prandial glucose (Table 5). In general these factors were also predictors of higher Δglucose and Δ% glucose. Clinical predictors of post-meal hyperglycaemia were essentially identical when post-prandial glucose >8.89 mmol/l, Δglucose ≥2.22 mmol/l and Δ% glucose ≥25 were modelled as outcome variables.
Discussion
Post-prandial hyperglycaemia may be a key metabolic disturbance contributing to the chronic complications of type 2 diabetes [1–4]. Despite the potential importance of post-prandial hyperglycaemia, its prevalence and correlates are incompletely understood. We examined the epidemiology of post-prandial hyperglycaemia in a very large, representative sample of non-insulin-treated type 2 diabetic patients and found that post-prandial hyperglycaemia and post-prandial glucose peaks were very common. The vast majority of patients recorded blood glucose exceeding the ADA and IDF thresholds for unacceptable hyperglycaemia (≥8.89 mmol/l) following a meal, and 40% of them had several exaggerated glucose excursions (Δglucose ≥2.22 mmol/l) in the post-prandial period. These values are consistent with data on post-OGTT hyperglycaemia collected in diabetic patients from NHANES [18], and underscore a deleterious metabolic abnormality that occurs commonly in everyday life and in a more typical physiological setting than an OGTT.
Importantly, post-prandial hyperglycaemia and post-prandial peaks were common even in subjects with an apparently good metabolic control (HbA1c <7%) and in subjects with average pre-prandial glucose levels within recommended goals (≤6.66 mmol/l; ≤120 mg/dl). Exaggerated post-prandial glucose excursions were less common in subjects with higher pre-prandial glucose levels than in those with lower glucose concentrations before the meals. Consistent with this result is the finding of an inverse correlation between pre-prandial glucose levels and absolute or relative glucose change after meals. These findings clearly document that the usual methods to assess glycaemic control are poorly informative of the capability of the single individual to control glucose excursions after meals, and neither HbA1c nor pre-prandial glucose measurement can reliably be expected to identify patients with acceptable post-prandial glucose levels or excursions. The logical conclusion is that for optimal glucose control to prevent diabetic complications, assessment of metabolic control cannot rely solely on periodical HbA1c measurements and that blood glucose self-monitoring cannot be limited to pre-prandial glucose readings. Our data suggest that the common habit of self-assessment of glycaemia at home only before meals [19] may need to be reconsidered and self-assessment of blood glucose after meals be added to routine self-monitored blood glucose policies. This approach meets guidelines of the IDF [12] and is consistent with current recommendations of the ADA [11].
We chose our definitions for post-prandial hyperglycaemia on the basis of treatment guidelines issued by the IDF and ADA. These guidelines indicate arbitrary thresholds of post-prandial hyperglycaemia. It is possible that lesser degrees of post-prandial hyperglycaemia (defined using a cut-off point <8.89 mmol/l) contribute to diabetes complications, although no data are available [10]. It is also possible that with the use of novel markers of post-meal glucose levels, such as depressed 1,5-anhydroglucitol levels [20], the prevalence of clinically significant post-prandial hyperglycaemia might be different. In addition, we based our definition of a deleterious post-prandial excursion (≥2.22 mmol/l) on risk associated with post-OGTT glucose levels [9]. Because post-meal and post-OGTT glucose excursions may be very different, depending on the constituents of the meal, it is possible that post-meal excursions may confer different risks from those of post-OGTT excursions, and that use of ≥2.22 mmol/l to define a deleterious post-prandial glucose excursion may either over- or underestimate the actual risk of complications. Interestingly, however, post-OGTT and post-meal glucose levels seems to be strongly correlated [21].
We also found that the contribution of pre- and post-prandial glucose to HbA1c was a function of prevailing HbA1c levels. When HbA1c was low, the main contribution came from post-prandial glucose, whereas when HbA1c was high, the main contribution came from pre-prandial glucose. This is consistent with data from Monnier et al. [16].
Data from many studies support the view that post-prandial glucose peaks appear to play a major role in the pathogenesis of chronic diabetic complications. In particular, complications related to accelerated atherosclerosis seem to be related to post-prandial peaks more strongly than to fasting glucose. This conclusion is based upon observational studies focusing on clinical CVD in diabetic patients who had an assessment of post-prandial glucose [5–7] as well as on data from large general population samples undergoing an OGTT [8, 9]. In addition, while intervention trials targeting fasting glucose and/or HbA1c exclusively have had at best a marginal effect on CVD in type 2 diabetes [22–24], trials specifically targeting post-prandial glucose appear to have been more effective and add further support to the hypothesis that post-prandial hyperglycaemia is a key contributor to diabetes complications [25, 26]. For instance, a post hoc analysis of an intervention trial using acarbose (which targets post-prandial hyperglycaemia) showed a 49% reduction in cardiovascular events in subjects with IGT [27]. More recently it has been reported that specifically controlling post-prandial hyperglycaemia using a rapid-acting secretagogue (repaglinide) compared with glyburide resulted in a similar improvement of HbA1c but was associated with a regression of carotid artery atherosclerosis [28]. In addition to these data, other clinical and experimental studies suggest that post-challenge hyperglycaemia or acute elevation of plasma glucose is deleterious for diverse functions related to atherogenesis [29–39]. Thus, the bulk of epidemiological, clinical and experimental evidence strongly suggests that efforts to monitor and control the common phenomenon of post-prandial hyperglycaemia should have uniquely beneficial effects on the chronic complications of type 2 diabetes.
Older age, longer duration of diabetes, absence of obesity, hypertension and dyslipidaemia are clinical features related to exaggerated post-prandial glucose peaks. These clinical features might be used to identify patients more likely to have post-prandial peaks and to need a careful post-prandial glucose assessment. Related to this observation we found that patients treated with sulfonylureas had greater post-prandial blood glucose excursions than subjects not taking these medications, suggesting that sulfonylureas are not able to prevent post-prandial glucose peaks. Patients taking sulfonylureas who have poor glycaemic control thus might benefit from therapy with glinides and short-acting insulin analogues to control post-prandial hyperglycaemia [40–46].
In recent studies carried out in a smaller number of subjects, Monnier and coworkers [15] observed that pre-lunch glucose concentration was higher than pre-breakfast and post-lunch glucose levels. We were not able to confirm this finding. On the contrary, we observed that glucose levels before the three meals were essentially identical. This means that, on average, non-insulin-treated type 2 patients are able to return to pre-meal glucose concentrations before the next meal, without a substantially progressive increase in blood glucose throughout the day despite additional meals. A corollary is that in most type 2 diabetic patients the treatment should specifically target early morning pre-breakfast hyperglycaemia on the one hand and post-prandial glucose peaks on the other hand. As a consequence, detailed glycaemic phenotyping of individual patients with careful home blood glucose monitoring is probably warranted to optimise glycaemic control. Assessment of post-prandial glycaemic excursions may be particularly helpful when the therapeutic plan is being re-evaluated to improve inadequate glycaemic control. Relatively simple schedules of home blood glucose monitoring can be prescribed on the basis of the individual clinical situation.
In conclusion, data from this large, representative sample of type 2 diabetes patients receiving routine care in diabetes clinics demonstrate that post-prandial hyperglycaemia is a very common phenomenon. Post-prandial hyperglycaemia occurs even when glycaemic control is apparently good according to HbA1c or fasting plasma glucose levels. Post-prandial hyperglycaemia can be predicted by simple clinical features, thus permitting identification of patients most likely to need a more complete glycaemic phenotyping. The assessment of post-prandial glucose levels and the evaluation of post-meal glucose excursions can establish the glycaemic phenotype and help to tailor the most appropriate treatment for individual patients, and is likely to contribute to improved glycaemic control for prevention of the devastating chronic complications of type 2 diabetes.
Abbreviations
- ADA:
-
American Diabetes Association
- CVD:
-
cardiovascular disease
- IDF:
-
International Diabetes Federation
References
Gerich JE (2003) Clinical significance, pathogenesis, and management of postprandial hyperglycaemia. Arch Intern Med 163:1306–1316
Haffner SM (1998) The importance of hyperglycaemia in the nonfasting state to the development of cardiovascular disease. Endocr Rev 19:583–592
Ceriello A (2003) The possible role of post-prandial hyperglycaemia in the pathogenesis of diabetic complications. Diabetologia 46 (Suppl 1):M9–M16
Bonora E, Muggeo M (2001) Post-prandial blood glucose as a risk factor for cardiovascular disease in type 2 diabetes. The epidemiological evidence. Diabetologia 44:2107–2114
Hanefeld M, Fischer S, Julius U et al (1996) Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia 39:1577–1583
Bonora E (2002) Postprandial peaks as a risk factor for cardiovascular disease: epidemiological perspectives. Int J Clin Pract Suppl 129:5–11
Cavalot F, Petrelli A, Traversa M et al (2005) Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women. J Clin Endocrinol Metab [Epub ahead of print]
The DECODE study group on behalf of the European Diabetes Epidemiology Group (2001) Glucose tolerance and cardiovascular mortality. Comparison of fasting and 2-h diagnostic criteria. Arch Intern Med 161:397–404
Meigs JB, Nathan DM, D’Agostino RB Sr, Wilson PW (2002) Fasting and post-challenge glycaemia and cardiovascular risk: the Framingham Offspring Study. Diabetes Care 25:1845–1850
American Diabetes Association (2001) Post-prandial blood glucose. Diabetes Care 24:775–778
American Diabetes Association (2005) Standard of medical care for patients with diabetes mellitus. Diabetes Care 28 (Suppl 1):S4–S40
European Diabetes Policy Group (1999) A desktop guide to type 2 diabetes mellitus. Diabetic Med 16:716–730
Bonora E, Calcaterra F, Lombardi S et al (2001) Plasma glucose levels throughout the day and HbA1c interrelationships in type 2 diabetes. Implications for treatment and monitoring of metabolic control. Diabetes Care 24:2023–2029
Avignon A, Radauceanu A, Monnier L (1997) Non-fasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 20:1822–1826
Monnier L, Colette C, Rabasa-Lhoret R et al (2002) Morning hyperglycaemic excursions. A constant failure in the metabolic control of non-insulin-using patients with type 2 diabetes. Diabetes Care 25:737–741
Monnier L, Lapinski H, Colette C (2003) Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycaemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 26:881–885
Meigs JB, Nathan DM, D’Agostino RB Sr, Wilson PW; Framingham Offspring Study (2002) Fasting and postchallenge glycaemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 25:1845–1850
Erlinger TP, Brancati FL (2001) Postchallenge hyperglycaemia in a national sample of US adults with type 2 diabetes. Diabetes Care 24:1734–1738
Harris MI, Cowie CC, Howie LJ (1993) Self-monitoring of blood glucose by adults with diabetes in the United States population. Diabetes Care 16:1116–1123
McGill JB, Cole TG, Nowatzke W et al (2004) Circulating 1,5-anhydroglucitol levels in adult patients with diabetes reflect longitudinal changes of glycaemia: a US trial of the GlycoMark assay. Diabetes Care 27:1859–1865
Wolever TMS, Chiasson JL, Csima A et al (1998) Variation of post-prandial plasma glucose, palatability and symptoms associated with a standardized mixed meal versus 75 g oral glucose. Diabetes Care 21:336–340
University Group Diabetes Program (1982) Effects of hypoglycaemic agents on vascular complications in patients with adult-onset diabetes. VIII. Evaluation of insulin therapy. Final report. Diabetes 31 (Suppl 5):1–26
Abraira C, Colwell JA, Nuttall FQ et al (1997) Cardiovascular events and correlates in the Veteran Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on glycemic control and complications in type 2 diabetes (VA CSDM). Arch Intern Med 157:181–188
UK Prospective Diabetes Study Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Malmberg K for the DIGAMI Study Group (1997) Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infraction in patients with diabetes mellitus. BMJ 314:1512–1515
Shichiri M, Kishikawa H, Ohkubo Y, Wake N (2000) Long term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 23 (Suppl 2):B21–B29
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP–NIDDM Trial Research Group (2003) Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP–NIDDM trial. JAMA 290:486–494
Esposito K, Giugliano D, Nappo F, Martella and the Campanian Postprandial Hyperglycaemia Study Group (2004) Regression of carotid atherosclerosis by control of postprandial hyperglycaemia in type 2 diabetes mellitus. Circulation 110:214–219
Pirags V, Assert R, Haupt K, Schatz H, Pfeiffer A (1996) Activation of human platelet protein kinase C-beta 2 in vivo in response to acute hyperglycaemia. Exp Clin Endocrinol Diabetes 104:431–440
Giugliano D, Marfella R, Coppola L et al (1997) Vascular effects of acute hyperglycaemia in humans are reversed by l-arginine: evidence for reduced availability of nitric oxide during hyperglycaemia. Circulation 95:1783–1790
Williams SB, Goldfine AB, Timimi FK et al (1998) Acute hyperglycaemia attenuates endothelium-dependent vasodilatation in humans in vivo. Circulation 97:1695–1701
Marfella R, Esposito K, Giunta R et al (2000) Circulating adhesion molecules in humans: role of hyperglycaemia and hyperinsulinemia. Circulation 101:2247–2251
Shige H, Ishikawa T, Suzukawa M et al (1999) Endothelium-dependent flow-mediated vasodilation in the post-prandial state in type 2 diabetes mellitus. Am J Cardiol 84:1272–1274
Kawano H, Motoyama T, Hirashima O et al (1999) Hyperglycaemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 34:146–154
Ceriello A, Giacomello R, Stel G et al (1995) Hyperglycaemia-induced thrombin formation in diabetes. The possible role of oxidative stress. Diabetes 44:924–928
Ceriello A, Taboga C, Tonutti L et al (2002) Evidence for an independent and cumulative effect of post-prandial hypertriglyceridemia and hyperglycaemia on endothelial dysfunction and oxidative stress generation: effect of short- and long-term simvastatin treatment. Circulation 106:1211–1218
Li W, Liu X, Yanoff M, Cohen S, Ye X (1996) Cultured retinal capillary pericytes die by apoptosis after an abrupt fluctuation from high to low glucose levels: a comparative study with retinal capillary endothelial cells. Diabetologia 39:537–547
Takeuchi A, Throckmorton DC, Brodgen AP, Yoshizawa N, Rasmussen H, Kashgarian M (1995) Periodic high extracellular glucose enhances production of collagens III and IV by mesangial cells. Am J Physiol 268:F13–F19
Porta M, Molinatti PA, Dosso AA, Williams FM, Brooks RA, Kohner EM (1994) Growth of bovine retinal pericytes and endothelial cells in high hexose concentrations. Diabetes Metab 20:25–30
Wolffenbuttel BHR, Landgraf R on behalf of the Dutch and German Repaglinide Study Group (1999) A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Diabetes Care 22:463–467
Gribble FM, Manley SE, Levy JC (2001) Randomized dose ranging study of the reduction of fasting and post-prandial glucose in type 2 diabetes by nateglinide. Diabetes Care 24:1221–1225
Horton ES, Clinkingbeard C, Gatlin M, Foley J, Mallows S, Shen S (2000) Nateglinide alone and in combination with metformin improves glycemic control by reducing mealtime glucose levels in type 2 diabetes. Diabetes Care 23:1660–1665
Fonseca V, Grunberger G, Gupta S, Shen S, Foley JE (2003) Addition of nateglinide to rosiglitazone monotherapy suppresses mealtime hyperglycaemia and improves overall glycemic control. Diabetes Care 26:1685–1690
Anderson JH, Brunelle R, Koivisto VA et al (1997) Insulin analogue treatment reduces post-prandial hyperglycaemia and frequency of hypoglycaemia in IDDM patients. Diabetes 46:265–270
Nielsen FS, Jorgensen LN, Ipsen M, Voldsgaard AI, Parving HH (1995) Long-term comparison of insulin analogue B10Asp and soluble human insulin in IDDM patients on a basal/bolus insulin regimen. Diabetologia 38:592–598
Ciofetta M, Lalli C, Del Sindaco P et al (1999) Contribution of post-prandial versus interprandial blood glucose to HbA1c in type 1 diabetes on physiologic intensive therapy with lispro insulin at mealtime. Diabetes Care 22:795–800
Acknowledgements
The enthusiastic collaboration of the physicians, nurses and patients participating in this multicentre study is gratefully acknowledged. The study was financially supported by Novartis. J. B. Meigs is supported by an American Diabetes Association Career Development Award.
Duality of interest E. Bonora has received unrestricted research support from Abiogen Pharma, GlaxoSmithKline and Novartis, and honoraria for consulting and speaking engagements from Abbott, Astrazeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Fournier Pharma, GlaxoSmithKline, Laboratori Guidotti, Merck Pharma, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi-Aventis, Servier and Takeda. A. Ceriello has received unrestricted research support from Amylin, Astrazeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Novartis, Novo Nordisk, Pfizer, Roche and Servier and honoraria for consulting and speaking engagement from Amylin, Astrazeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Lifescan, Laboratori Guidotti, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Servier and Takeda. J. B. Meigs has received unrestricted research support from Sanofi-Aventis, GlaxoSmithKline, Novartis, Pfizer and Wyeth, and has served on safety monitoring or advisory boards for GlaxoSmithKline, Eli Lilly, Pfizer and Merck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonora, E., Corrao, G., Bagnardi, V. et al. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia 49, 846–854 (2006). https://doi.org/10.1007/s00125-006-0203-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0203-x