Abstract
Aim/hypothesis
Type I diabetes is associated with altered hepatic bile formation and increased intestinal cholesterol absorption. The aim of this study was to evaluate whether altered expression of the ATP-Binding Cassette half-transporters Abcg5 and Abcg8, recently implicated in control of both hepatobiliary cholesterol secretion and intestinal cholesterol absorption, contributes to changed cholesterol metabolism in experimental diabetes.
Methods
mRNA and protein expression of Abcg5 and Abcg8 were determined in the liver and intestine of rats with streptozotozin-induced diabetes and related to relevant metabolic parameters in plasma, liver and bile.
Results
Hepatic mRNA expression of both Abcg5 (−76%) and Abcg8 (−71%) was reduced in diabetic rats when compared to control rats. In spite of increased HDL cholesterol, considered a major source of biliary cholesterol, secretion of the sterol into bile relative to that of bile salts was reduced by 65% in diabetic animals. Intestinal mRNA expression of Abcg5 (−47%) and Abcg8 (−43%) as well as Abcg5 protein contents were also reduced in insulin-deficient animals. This was accompanied by a three- to four-fold increase in plasma β-sitosterol and campesterol concentrations and by a doubling of the calculated apparent cholesterol absorption. These effects partially normalized upon insulin supplementation.
Conclusion/interpretation
Our data indicate that effects of insulin-deficiency on bile composition and cholesterol absorption in rats are, at least partly, attributable to changes in hepatic and intestinal Abcg5 and Abcg8 expression.
Similar content being viewed by others
Type I diabetes mellitus is associated with specific changes in cholesterol metabolism in humans [1] and in experimental animals [2, 3], including increased concentrations of plasma cholesterol, enhanced conversion of cholesterol into bile salts and an enhanced intestinal cholesterol absorption. The hepatobiliary pathway is of crucial importance for the maintenance of cholesterol homeostasis [4]. Bile salts that are secreted by the liver into the intestinal lumen are required for intestinal absorption of dietary cholesterol. The majority of bile salts is subsequently reabsorbed from the intestine and returns to the liver for re-secretion into the bile. The relatively small fraction of bile salts that escapes intestinal absorption is compensated for by de novo synthesis from cholesterol in the liver. Secondly, bile contains considerable amounts of free cholesterol. Since only a part of biliary cholesterol is reabsorbed from the intestine [5], the biliary pathway contributes to a major extent to cholesterol turnover. It is well-established that secretion of cholesterol into bile is coupled to that of phospholipids in a process that is, in part, controlled by bile salt secretion [6]. Recent studies, however, indicate that specific ABC transporters, i.e., Abcg5 and Abcg8, are involved in biliary cholesterol secretion [7, 8]. The genes encoding these transporters are highly expressed in the liver [9]. Mutations in the human genes encoding ABCG5 and ABCG8 have been shown to cause sitosterolaemia [10, 11, 12] with a reduced biliary secretion as well as a strongly enhanced intestinal absorption of plant sterols (sitosterol, campesterol). Indeed, ABCG5 and ABCG8 are also highly expressed in the intestine [9] and supposedly involved in efflux of plant sterols taken up by enterocytes back into the intestinal lumen, thereby preventing absorption. Based on the fact that the efficiency of dietary cholesterol absorption is high in sitosterolaemia patients, a role of ABCG5 and ABCG8 in the control of cholesterol absorption efficiency has been proposed [10, 11, 12]. Accordingly, cholesterol absorption was reduced in mice over-expressing both transporters [7] and in mice in which expression of the transporters was induced by pharmacological means [13].
Type I diabetes is associated with altered expression of several ABC transporters in the liver. In a recent study [14], we showed that streptozotocin (STZ)-induced diabetes in rats differentially affects the expression of hepatic ABC transporters which, at least in part, underlie reported effects on bile composition. Specifically, we found a very strong up-regulation of Abcb4 (multidrug resistance P-glycoprotein subtype 2 or Mdr2) mRNA and Abcb4 protein, in accordance with a strong induction of biliary phospholipid secretion. In spite of the characteristic increase in biliary bile salt output rates, we found no effects on Abcb11 (bile salt export pump or Bsep) protein content in livers of STZ-diabetic rats.
To investigate whether changes in Abcg5/Abcg8 expression contribute to the established effects of insulin deficiency on cholesterol metabolism, we have determined their mRNA abundances and protein contents in the liver and intestine of rats with STZ-diabetes and related these to the actual sterol fluxes. Our data indicate that the suppressive effects of insulin-deficiency on biliary cholesterol secretion and its stimulatory effects on cholesterol absorption in rats are, at least in part, attributable to changes in hepatic and intestinal Abcg5 and Abcg8 expression.
Materials and methods
Animals
Male Wistar rats (260–300 g) were purchased from Harlan (Zeist, The Netherlands) and housed in a temperature-controlled environment with alternating 12-h light and dark periods. The rats received standard laboratory chow (RMH-B; Hope Farms BV, Woerden, The Netherlands) and had free access to food. Experimental procedures were approved by the local Ethics Committee for Animal Experimentation.
Experimental procedures
Diabetes was induced by a single intraperitoneal injection (60 mg/kg body weight) of STZ (Pharmacia & Upjohn, Kalamazoo, Mich., USA). Control animals received an injection of the solvent (sodium citrate, 3% w/v). Induction of diabetes was perceived by development of hyperphagia, polydipsia and polyuria and confirmed by determination of the degree of hyperglycaemia. Three weeks after STZ injection, one half of the diabetic group was treated with subcutaneously administered insulin (long acting insulin, Humuline NPH, Eli Lilly, Nieuwegein, The Netherlands, 1 IU in the morning and 2 IU in the evening). Experiments were carried out at 4 weeks after STZ injection. Food intake was monitored by weighing of food containers and faeces was collected quantitatively during the last 3 days prior to death of the animals. At that time, six control, six diabetic and six diabetic insulin-treated rats were anaesthetized with pentobarbital (60 mg/kg body weight) and bile was collected for 30 min upon cannulation of the bile duct. Blood samples were collected by heart puncture, transferred to EDTA-containing tubes and centrifuged immediately (10 000 g). Plasma was stored at –20°C until analyses and the livers were rapidly excised and weighed. Parts of the liver were snap-frozen in liquid nitrogen for RNA isolation, isolation of plasma membrane fractions or determination of lipid concentrations. The small intestines were flushed with a buffered salt solution; representative parts of the intestine were snap-frozen in liquid nitrogen for RNA isolation or isolation of brush border membranes.
Analytical procedures
Plasma concentrations of total cholesterol, triglycerides and free fatty acids were measured with commercially available kits (Roche, Mannheim, Germany, or Wako, Neuss, Germany) [13, 15]. Pooled plasma samples of the three groups of rats were used for lipoprotein separation by fast protein liquid chromatography (FPLC) [15]. Plasma plant sterol and cholesterol concentrations were determined by gas chromatography [16]. Biliary bile salt concentrations were measured enzymatically [13, 15]. Hepatic and biliary phospholipids and cholesterol lipid contents were measured after extraction [17, 18, 19]. Faecal and chow contents of neutral sterols were measured as described [15].
Western blotting
Plasma lipoprotein fractions separated by FPLC were taken for semi-quantitative assessment of apoA-I contents by Western blotting [15].
Hepatic plasma membrane fractions were prepared and characterized as described [20]. Proteins from liver homogenates (75 µg protein / lane) or liver plasma membranes (10 µg protein/lane) were separated on 4–15% Ready Gels (Bio-Rad Laboratories, Hercules, Calif., USA) and blotted onto nitrocellulose membranes by tank blotting. Membranes were blocked overnight in a 5% skimmed milk powder solution in Tris-buffered saline containing 0.1% Tween 20 (TTBS) and subsequently incubated with the primary antibody (rabbit polyclonal anti-SR-BI, Novus Biologicals, Littleton, Colo., USA, NB400–101) diluted 1:20 000 in TTBS for 1 h at room temperature. After washing, anti-rabbit IgG linked to horse radish peroxidase, diluted 1:1000 in TTBS, was added for 1 h. Detection was carried out using ECL, according to the manufacturer’s instructions (Amersham, Roosendaal, the Netherlands).
Proteins from intestinal brush border membranes, isolated as described previously [20], were separated on 8% SDS-polyacrylamide gels and blotted onto nitrocellulose membranes. Membranes were blocked in TTBS and subsequently incubated for 1 h at room temperature with primary antibodies, raised in rabbits against amino acids 256–392 of murine Abcg5 [21], diluted 1:1000 in blocking buffer. Membranes were washed thrice in TTBS and incubated with horseradish peroxidase-conjugated goat anti-rabbit antibodies (Bio-Rad) diluted 1:2000 in blocking buffer. Membranes were washed four times in TTBS and bands were visualized using Lumi-lightplus Western blotting substrate in a Lumi-Imager F1 workstation (Roche).
RNA isolation and RT-PCR procedures
RNA isolation and cDNA synthesis were carried out as described [22]. Real-time quantitative PCR was done as described [23] and modified in our laboratory [13]. Primer and probe sequences (Invitrogen, Carlsbad, USA) and detection probes (Eurogentec, Seraing, Belgium) for the genes of interest, labelled with the 5′ linked fluorescent reporter dye 6-carboxy-fluorescein (FAM) and the 3′ linked fluorescent quenching dye 6-carboxy-tetramethyl-rhodamine (TAMRA) [13]. Measurements were done using an ABI Prism 7700 Sequence Detector with 1.6.3 software (Perkin-Elmer Corp., Foster City, Calif., USA).
Statistics
Results are presented as mean values±SD. Statistic analyses were carried out using one-way ANOVA with Bonferroni correction or, when two groups were compared, by the Mann-Whitney U test. A p value of less than 0.05 was considered statistically significant.
Results
Animal characteristics
STZ rats had lower body weights than control rats at the end of the experiment (−29%, p<0.05), which did not normalize upon treatment with insulin (−25%). The ratio liver-to-body weight was increased by 37% (p<0.05) upon STZ treatment and this effect did not disappear after insulin treatment. Blood glucose concentrations were higher in diabetic rats than in controls, i.e., 23.2±3.5 compared with 5.8±0.3 mmol/l (p<0.05), whereas those in insulin-treated diabetic rats were intermediate (11.9±5.4 mmol/l).
Plasma cholesterol, triglyceride, and free fatty acid concentrations were all increased in diabetic rats and showed a tendency towards normalization in insulin-treated diabetic rats (Table 1). FPLC separation of plasma lipoproteins showed that the increase in plasma cholesterol in diabetic animals was due to increases in VLDL-, LDL- as well as HDL-sized fractions (Fig. 1A), whereas the increase in triglycerides was exclusively in VLDL-sized fractions (Fig. 1B). Western blot analysis of FPLC fractions revealed a higher apolipoprotein (apo) A-I content in HDL fractions of diabetic rats than in those of control rats (Fig. 1C). Hepatic mRNA abundance of Apoa-I was more than two-fold induced in diabetic rats and normalized upon insulin treatment (data not shown).
Effects of streptozotocin-induced diabetes on distribution of cholesterol (a), triglycerides (b) and apolipoprotein A-I (c) in plasma lipoprotein fractions Open symbols, control rats; closed symbols, diabetic rats. Diabetic rats treated with insulin showed concentrations of cholesterol and triglycerides that were intermediate between those of controls and untreated diabetic rats across all lipoprotein fractions: these data are not shown for reasons of clarity. Top panel c, control rats; bottom panel c, diabetic rats
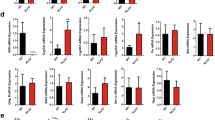
No differences in hepatic free cholesterol contents were noted between control, diabetic and insulin-treated diabetic animals, i.e., 25.5±1.4, 24.0±3.6 and 25.5±4.5 nmol/mg protein, respectively. As expected, expression of sterol regulatory element binding protein 1c (Srebp1c) was strongly reduced in diabetic animals whereas that of Srebp2, the transcription factor that is primarily responsible for control of genes involved in maintenance of hepatocytic cholesterol homeostasis, remained unaffected (Fig. 2). Expression of genes encoding proteins involved in cholesterol synthesis (HMG CoA reductase, Hmgr) and lipoprotein uptake (LDL receptor, Ldlr) were down-regulated in diabetic animals, whereas that of acyl-CoA cholesterol acyltransferase 2 (Acat2), involved in cholesterol esterification, was not affected (Fig. 2).
Changes in relative hepatic gene expression of Srebp1c, Srebp2, Hmgr, Ldlr, and Acat 2 upon induction of streptozotocin-diabetes, determined by realtime PCR Open bars, control rats; closed bars, diabetic rats; striped bars; diabetic rats treated with insulin. Mean values±SD of 4–6 rats per group, asterisks indicate significant difference from control values
Reduced hepatic expression of Abcg5 and Abcg8 is associated with impaired hepatobiliary cholesterol transport
mRNA abundances of Abcg5 and Abcg8 were strongly reduced in the livers of diabetic rats in comparison to those in control animals. This consequence of long-term insulin-deficiency did not normalize upon treatment of diabetic rats with insulin. Both Abcg5 and Abcg8 gene expression are controlled by the liver X-receptor: no differences in expression of the gene encoding the most abundant isoform of this transcription factor in the liver, i.e., Lxrα, were noted between the groups (Fig. 3).
Changes in relative hepatic gene expression of Lxrα, Abcg5 and Abcg8 upon induction of streptozotocin-diabetes in rats, determined by realtime PCR. Open bars, control rats; closed bars, diabetic rats; striped bars; diabetic rats treated with insulin. Mean values±SD of six rats per group, asterisks indicate significant difference from control values
To test the functional consequences of reduced hepatic Abcg5 and Abcg8 expression, we measured biliary cholesterol output in rats of the three experimental groups. Cholesterol output in the three groups of animals was expressed relative to that of bile salts (Fig. 4A) and to that of phospholipids (Fig. 4B). It is evident that diabetic rats secreted much less cholesterol relative to bile salts and to phospholipids than control rats did: insulin treatment failed to completely restore hepatobiliary cholesterol hyposecretion.
Changes in biliary cholesterol content upon induction of streptozotocin-diabetes in rats. Biliary cholesterol concentration was expressed relative to that of bile salts (a) or phospholipids (b). Open bars, control rats; closed bars, diabetic rats; striped bars; diabetic rats treated with insulin. Mean values±SD of six rats per group, asterisks indicate significant difference from control values
HDL is considered to be an important source of biliary cholesterol and concentrations of plasma HDL cholesterol were clearly elevated in diabetic rats (Fig. 1). To evaluate whether a reduced hepatic uptake capacity of HDL cholesterol (ester) might contribute to biliary cholesterol hyposecretion, we analysed hepatic mRNA and protein expression of the major HDL-receptor, i.e., scavenger receptor class B type 1 (SR-BI) in livers of the three groups of rats. mRNA abundance of Sr-bI was slightly higher in diabetic rats than in controls and normalized upon insulin treatment (Fig. 5). SR-BI protein content was clearly increased in liver homogenates of diabetic rats compared to those of controls and insulin-treated diabetic rats (Fig. 5A) while the amounts of the protein in hepatic plasma membrane fractions were rather similar among the three groups (Fig. 5B).
Effects of streptozotocin-diabetes on hepatic mRNA and protein expression of SR-BI. Open bars, control rats; closed bars, diabetic rats; striped bars; diabetic rats treated with insulin. Mean values±SD of six rats per group, asterisks indicate significant difference from control values. Inserts show corresponding levels of SR-BI protein in liver homogenates (a) and plasma membrane fractions (b). Data shown are representative examples of at least four preparations per group. C, control; D, diabetics; D + I, diabetics + insulin
Reduced intestinal expression of Abcg5 and Abcg8 is associated with enhanced cholesterol absorption
mRNA expression levels of Abcg5 and Abcg8 were reduced by 47% and 43% in the jejunum of diabetic rats when compared to controls and normalized upon insulin treatment (Fig. 6). The expression of Abca1, also implicated in the control of intestinal cholesterol absorption, showed a similar pattern as those of Abcg5 and Abcg8. Intestinal expression of Lxrα tended to be reduced in diabetic rats and normalized upon insulin treatment.
Changes in relative intestinal gene expression of Lxrα, Abcg5, Abcg8 and Abca1, determined by realtime PCR, and intestinal Abcg5 protein content upon induction of streptozotocin-diabetes in rats. Open bars, control rats; closed bars, diabetic rats; striped bars; diabetic rats treated with insulin. Mean values±SD of six rats per group, asterisks indicate significant difference from control values, #indicates p=0.059. Insert shows Abcg5 protein levels in brush border membranes from jejunal sections of rats of the three groups. Bands at 90 and 75 kD reacted to the antibody raised against mouse Abcg5. Competition with the peptide used to raise the antibody strongly decreased these signals. Incubation of the protein fractions with N-glycosidase F to remove all N-linked sugar chains decreased the apparent molecular weight of both bands to a single band of ~64 kD. C, control; D, diabetics; D + I, diabetics + insulin
Reduced mRNA abundance was associated with a clearly reduced protein content of Abcg5 in brush border membrane preparations isolated from the small intestines of diabetic rats (Fig. 6).
To assess the functional consequences of reduced Abcg5 and Abcg8 expression in the intestines of diabetic rats, plasma concentrations of the plant sterols campesterol and β-sitosterol were determined. These sterols are considered natural substrates of Abcg5/Abcg8: their concentrations are strongly increased in sitosterolaemia patients and have been advocated as an indirect measure of cholesterol absorption efficiency. The concentration of both sterols was increased in diabetic rats when compared to control values and decreased upon insulin treatment of diabetic animals (Fig. 7A). Plasma plant sterol concentrations remained increased in diabetic rats when normalized to plasma cholesterol concentrations (Fig. 7B). The difference between calculated daily biliary excretion rates of cholesterol and an estimation of dietary cholesterol intake (input) and daily faecal neutral sterol loss (output) were used to calculate the “apparent cholesterol absorption” in the three groups of animals (Fig. 7C). It was found that this calculated value was increased in the diabetic animals in comparison to controls, while it decreased again after treatment of diabetic animals with insulin.
Changes in indices of intestinal cholesterol absorption efficiency upon induction of streptozotocin-diabetes in rats. Plasma concentrations of campesterol and β-sitosterol were measured in control rats, diabetic rats and diabetic rats treated with insulin (a) and normalized to that of cholesterol, to exclude that the observed changes in plasma plant sterols were due to a-selective alterations in plasma sterol levels (b). Apparent cholesterol absorption was calculated for the three groups by subtraction of the measured daily faecal neutral sterol loss from the estimated daily input (c). Open bars, control rats; closed bars, diabetic rats; striped bars; diabetic rats treated with insulin. Mean values±SD of six rats per group, asterisks indicate a significant difference from control values, & indicates significant difference from diabetes group
Discussion
The results show that STZ-induced Type I diabetes in rats is associated with reduced hepatic and intestinal expression of the ABC half-transporters Abcg5 and Abcg8. Reduced hepatic expression of these “sitosterolaemia genes” coincided with a reduction of hepatobiliary cholesterol secretion, whereas their reduced intestinal expression was associated with an increased absorption of cholesterol as deduced from an indirect measure of the process (plasma plant sterol concentrations) and from calculation of the apparent absorption efficiency. Similar changes in cholesterol transport have been reported in sitosterolaemia patients [24] and in Abcg5/Abcg8-deficient mice [8]. Over-expression of the human genes in transgenic mice [7] and pharmacological induction of expression of the endogenous genes in wild-type mice [13] have been reported to have opposite effects, i.e., to stimulate biliary cholesterol excretion and to reduce intestinal cholesterol absorption. Consequently, it is highly likely that effects of insulin-deficiency on cholesterol metabolism are, at least in part, caused by changes in Abcg5 and Abcg8 expression.
Recently, two groups have independently identified mutations in either ABCG5 or ABCG8 as the cause of the rare, recessively inherited metabolic disease sitosterolaemia [10, 11, 12]. Patients with this disease develop xanthomas and premature atherosclerosis [24, 25]. Affected individuals show high concentrations of plant sterols in plasma due to the fact that, in contrast to healthy subjects, they efficiently absorb these sterols from the intestine and are unable to excrete them into the bile [24, 26]. Sitosterolaemia patients have also been reported to efficiently absorb dietary cholesterol and to show impaired biliary cholesterol excretion [26, 27]. ABCG5/Abcg5 and ABCG8/Abcg8 are predominantly expressed in hepatocytes and in small intestinal enterocytes in humans and mice. The two genes are arranged in a head-to-head configuration in the human [28] and mouse [9] genome. Expression of both genes is co-ordinately regulated and highly induced in mice kept on a high-cholesterol diet [29]. LXRα, a nuclear receptor activated by oxysterols that plays a crucial role in regulating genes involved in cholesterol trafficking [30], is required for induction of murine Abcg5 and Abcg8 expression upon cholesterol feeding [29]. Treatment of mice with synthetic LXR agonists strongly induces the expression of both genes in liver and intestine [13, 29]. Recent studies [31], in which epitope-tagged mouse Abcg5 and Abcg8 were expressed in cultured cells, have shown that heterodimerization of these half-transporters is required for their transport from the endoplasmic reticulum to the apical plasma membrane. Thus, available data indicate that the Abcg5/Abcg8 heterodimer is present at the canalicular membrane of hepatocytes where it is involved in secretion of cholesterol and plant sterols into the bile. In the intestine, the heterodimer seems to promote the efflux of (dietary) sterols, taken up by the enterocytes by as yet unidentified mechanisms, back into the lumen and thereby reduce the efficiency of their absorption. Overall, the physiological action of the transporter complex limits accumulation of sterols in the body.
Under normal conditions, biliary secretion of cholesterol is tightly coupled to that of phospholipids in a process controlled by bile salt secretion [6]. Accordingly, one would expect biliary cholesterol secretion to be enhanced in diabetic rats. This was evidently not the case: in spite of a strong increase in biliary bile salt and phospholipid secretion [14], diabetic rats displayed a relative hyposecretion of biliary cholesterol. Theoretically, hyposecretion could be explained by a lack of bile-destined cholesterol in the liver. It has been proposed that HDL cholesterol is a primary source of biliary cholesterol after its selective uptake by SR-BI [32]. One study [33] reported biliary cholesterol hyposecretion in SR-BI-deficient mice while another [34] reported hypersecretion in mice with hepatic SR-BI over-expression. We found that plasma HDL cholesterol and apo A-I levels were increased in diabetic rats, as is the case in SR-BI-deficient mice [33]. Yet, hepatic Sr-bI mRNA expression was up-regulated and corresponding protein contents were clearly increased in liver homogenates. SR-BI protein remained largely unaffected in plasma membrane fractions isolated from livers of diabetic rats, suggestive for altered sorting. Taken together, our data do not support impaired SR-BI-mediated HDL uptake as a cause of cholesterol hyposecretion in diabetic rats. In fact, our recent observation that cholesterol secretion is unaffected in Abca1 null mice lacking HDL [35] strongly argues against a regulatory role of cholesterol delivery. A concise overview of various models of cholesterol hypo- and hypersecretion indicated that, at least in mice, biliary cholesterol secretion strongly correlates with hepatic Abcg5/Abcg8 expression [21]. Accordingly, we propose that impaired hepatic expression of both half-transporters underlies impaired cholesterol secretion in diabetic rats. Insulin treatment of diabetic rats did not normalize hepatic Abcg5/Abcg8 expression and, consequently, failed to normalize cholesterol secretion into bile.
The intestine is another site were Abcg5/Abcg8 exert control on cholesterol metabolism, i.e., by regulating the efficiency of cholesterol absorption. It is known that cholesterol absorption is increased in chronically diabetic rats [2, 3], but underlying mechanisms have remained elusive so far. Our study confirms the increased cholesterol absorption in STZ-treated rats by two independent methods. We found that plasma concentrations of β-sitosterol and campesterol were three- to four-fold higher in diabetic rats than in controls and tended to normalize upon treatment of diabetic rats with insulin. Furthermore, we have calculated the apparent absorption by subtracting daily faecal neutral sterol output from the estimated daily input of dietary and biliary cholesterol. Although this calculation is based on a number of assumptions, the results strongly suggest that the apparent absorption is increased in diabetic animals. Intestinal hypertrophy [36] and the enlarged bile salt pool [3] have been proposed as rather unspecific causes of enhanced cholesterol absorption in experimental diabetes. Furthermore, cholesterol esterification by the enzyme acyl-CoA cholesterol acyltransferase, producing cholesteryl esters that can be incorporated into chylomicrons, has been shown to be enhanced in diabetic animals [37]. Our data show that reduced expression of Abcg5 and Abcg8 in the intestine of diabetic rats could contribute to the enhanced cholesterol absorption associated with this condition.
The obvious question concerns the cause of Abcg5/Abcg8 down-regulation in liver and intestine of insulin-deficient rats. As mentioned previously, both genes are under control of LXRα, a sterol-sensing nuclear receptor that, upon dimerization with activated RXR, induces transcription of a large number of genes involved in cholesterol trafficking [30]. We found no changes in mRNA expression of Lxrα itself or of Rxrα , either in the liver or intestine. Lxrα expression was reported to be induced in cultured hepatocytes exposed to insulin and in livers of rats and mice upon acute administration of insulin [38]. This suggests that, in our chronic model of insulin deficiency, alternative modes of regulation maintain hepatic Lxrα expression. This obviously does not exclude the possibility of reduced LXRα protein concentrations or reduced amounts of its most potent ligands, i.e., (24S), 25-epoxycholesterol, (24S)-hydroxycholesterol, or (22R)-hydroxycholesterol. Alternatively, it could be that metabolic consequences of diabetes interfere with LXRα signalling. In a recent paper [39], evidence was provided to suggest that reduced expression of another important ABC transporter, i.e., Abca1, in liver and macrophages of diabetic mice is due to the characteristically high concentrations of free fatty acids and ketone bodies (particularly acetoacetate). In this study, we have confirmed down-regulation of Abca1 expression in the liver of diabetic rats and show that expression of this gene is also strongly reduced in their small intestine. Expression of Abca1 is also under control of LXRα [30] and unsaturated fatty acids have been shown to antagonize activation of this nuclear receptor by oxysterols so that transcription of target genes is inhibited [40, 41]. The mechanism by which acetoacetate interferes with ABC transporter expression is fully elusive at the moment but, in view of the similar mode of regulation of Abca1 and Abcg5/Abcg8 genes [30], it could well be involved in the down-regulation of Abcg5/Abcg8 observed in this study. Thus, impaired Abcg5/Abcg8 expression in liver and intestine in diabetes may be related to the accelerated lipolysis and/or increased ketogenesis that is associated with this condition. A direct role of insulin-deficiency per se is not likely, since we found no differences in Abcg5/Abcg8 expression between rat hepatocytes cultured for up to 24 h in the absence or presence of insulin.
In conclusion, we have provided evidence that suppression of Abcg5 and Abcg8 expression in the liver and intestine contributes to altered hepatobiliary and intestinal sterol fluxes that collectively promote accumulation of these sterols in the body in diabetic rats. When similar events occur in human diabetic patients, as suggested by increased plasma levels of plant sterols in subjects with poorly controlled Type I diabetes [42], this could contribute to an enhanced risk for development of atherosclerosis [43].
Abbreviations
- ABC:
-
ATP-Binding Cassette
- Bsep:
-
bile salt export pump
- ECL:
-
enhanced chemoluminesence
- FPLC:
-
fast protein liquid chromatography
- Mdr:
-
multidrug resistance gene
- PCR:
-
polymerase chain reaction
- STZ:
-
streptozotocin
References
Bennion LJ, Grundy SM (1979) Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med 296:1365–1371
Young NL, Lopez DR, McNamara DJ (1988) Contributions of absorbed dietary cholesterol and cholesterol synthesized in small intestine to hypercholesterolemia in diabetic rats. Diabetes 37:1151–1156
Young NL, McNamara DJ, Krasovsky J, Lopez DR, Levy G (1983) Hyperphagia alters cholesterol dynamics in diabetic rats. Diabetes 32:811–819
Dietschy JM, Turley SD, Spady DK (1993) Role of liver in maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res 34:1637–1659
Wilson MD, Rudel LL (1994) Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J Lipid Res 35:943–955
Verkade HJ, Vonk RJ, Kuipers F (1995) New insights into the mechanism of bile acid-induced biliary lipid secretion. Hepatology 21:1174–1189
Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton, JD, Cohen JC, Hobbs HH (2002) Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 110:671–680
Yu L, Hammer RE, Li-Hawkins J, von Bergman K, Lutjohan D, Cohen JC, Hobbs HH (2002) Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA 16237–16242
Lu K, Lee M-H, Yu H, Zhou Y, Sandell SA, Salen G, Patel SB (2002) Molecular cloning, genomic organization, genetic variations, and characterization of murine sterolin genes Abcg5 and Abcg8. J Lipid Res 43:565–578
Lee M-H, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB (2001) Identification of a gene, ABCG5, important in the regulation of cholesterol absorption. Nat Gen 27:79–83
Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH (2000) Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290:1771–1775
Lu K, Lee M-H, Hazard A, Brooks-Wilson A, Hidaka H, Kojima H, Ose L, Stalenhoef AFH, Mietinen T, Bjorkhem I, Brukert E, Pandya A, Brewer HB Jr, Salen G, Dean M, Srivastava A, Patel SB (2001) Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8 respectively. Am J Hum Gen 69:278–290
Plösch T, Kok T, Bloks VW, Smit MJ, Havinga R, Chimini G, Groen AK, Kuipers F (2002) Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X-receptor (LXR) is independent of ABCA1. J Biol Chem 277:33870–33877
Van Waarde WM, Verkade HJ, Wolters H, Havinga R, Baller J, Bloks VW, Müller M, Sauer PJJ, Kuipers F (2002) Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC transporters in rats. Gastroenterology 122:1842–1852
Voshol PJ, Havinga R, Wolters H, Ottenhoff R, Princen HMG, Oude Elferink RPJ, Groen AK, Kuipers F (1998) Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P-glycoprotein-deficient mice. Gastroenterology 114:1024–1034
Kuksis A, Myher JJ, Marai L, Little JA, McArthur RG, Roncari DA (1986) Usefulness of gas chromatographic profiles of plasma total lipids in diagnosis of phytosterolemia. J Chromatogr 381:1–12
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochim Biophys 37:911–917
Böttcher CFJ, Gent CM van, Pries C (1961) A rapid and sensitive sub-micro-phosphorus determination. Anal Chim Acta 24:203–204
Gamble W, Vaughan M, Kruth MS, Avigan J (1978) Procedure for determination of free and total cholesterol in micro- and nanogram amounts suitable for studies with cultured cells. J Lipid Res 19:1068–1071
Wolters H, Spiering M, Gerding A, Slooff MJH, Kuipers F, Hardonk MJ, Vonk RJ (1991) Isolation and characterization of canalicular and basolateral plasma membrane fractions from human liver. Biochim Biophys Acta 1069:61–69
Kosters A, Frijters RJJM, Schaap FG, Vink E, Plösch T, Ottenhoff R, Jirsa M, De Cuyper IM, Kuipers F, Groen AK (2003) Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J Hepatol 38:710–716
Bloks VW, Plösch T, Goor H van, Roelofsen H, Baller J, Havinga R, Verkade HJ, Tol A van, Jansen PLM, Kuipers F (2001) Hyperlipidemia and atherosclerosis associated with liver disease in ferrochelatase-deficient mice. J Lipid Res 42:41–50
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6:986–994
Salen G, Shefer S, Nguyen L, Ness GC, Tint S, Shore V (1992) Sitosterolemia. J Lipid Res 33:945–955
Lee MH, Lu K, Patel SB (2001) Genetic basis of sitosterolemia. Curr Opin Lipidol 12:141–149
Salen G, Shore V, Tint S, Forte T, Shefer S, Horak I, Horak E, Dayal B, Nguyen L, Batta AK (1989) Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res 30:1319–1330
Bhattacharyya AK, Connor WE, Lin DS, McMurry MM, Shulman RS (1991) Sluggish sitosterol turnover and hepatic failure to excrete sitosterol into bile cause expansion of body pool of sitosterol in patients with sitosterolemia and xanthomatosis. Arterioscler Thromb 11:1287–1294
Remaley AT, Bark S, Walts AD, Freeman L, Shulenin S, Annilo T, Elgin E, Rhodes HE, Joyce C, Dean M, Santamaria-Fojo S, Brewer HB Jr (2002) Comparative genome analysis of potential regulatory elements in the ABCG5-ABCG8 gene cluster. Biochem Biophys Res Commun 295:276–282
Repa JJ, Berge KE, Pomajzl C, Richardson, Hobbs H, Mangelsdorf DJ (2002) Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors α and β. J Biol Chem 277:18793–18800
Repa JJ, Mangelsdorf DJ (2002) The liver X receptor gene team: potential new players in atherosclerosis. Nat Med 8:1243–1248
Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH (2002) Coexpression of ATP binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest 110:659–669
Ji Y, Wang N, Ramakrishnan R, Sehayek E, Huszar D, Breslow JL, Tall AR (1999) Hepatic scavenger receptor BI promotes rapid clearance of high density lipoprotein free cholesterol and its transport into bile. J Biol Chem 274:33398–33402
Mardones P, Quinones V, Amigo L, Moreno M, Miquel JF, Schwarz M, Miettinen HE, Krieger M, VanPatten S, Cohen DE, Rigotti A (2001) Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res 42:170–180
Kozarsky KF, Donahee MA, Rigotti A, Iqbal SN, Edelman ER, Krieger M (1997) Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 387:414–417
Groen AK, Bloks VW, Bandsma RHJ, Ottenhoff R, Chimini G, Kuipers F (2001) Hepatobiliary cholesterol transport is not impaired in ABCA1 null mice lacking high density lipoproteins. J Clin Invest 108:843–850
Schedl HP, Wilson HD (1971) Effects of diabetes on intestinal growth in the rat. J Exp Zool 176:487–496
Suckling KE, Stange EF (1985) Role of acylCoA cholesterol acyltransferase in cellular cholesterol metabolism. J Lipid Res 26:647–671
Tobin KAR, Ulven SM, Schuster GU, Hermansen Steiniger H, Andresen SM, Gustafsson JA, Nebb HI (2002) Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J Biol Chem 277:10691–10697
Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, Langer C, Schachtrup C, Wiekowski J, Lorowski S, Assmann G, von Eckardstein A (2002) Polyunsaturated fatty acids and acetoacetate downregulate the expression of the ATP-binding cassette transporter A1. Diabetes 51:2922–2982
Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y, Goldstein JL, Brown MS (2001) Unsaturated fatty acids inhibit transcription of the sterol regulatory element binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent acitvation of the LXR. Proc Natl Acad Sci USA 98:6027–6032
Yoshikawa T, Shimano H, Yahagi N, Ide T, Amamiya-kudo M, Matsuzaka T, Nakakuki M, Tomita S, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Takahashi A, Sone H, Osuga Ji J, Gotoda T, Ishibashi S, Yamada N (2002) Polyunsaturated fatty acids suppress sterol regulatory elemnent-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J Biol Chem 277:1705–1711
Kojima H, Hidaka H, Matsumura K, Fujita Y, Yamada S, Haneda M, Yasuda H, Kikkawa R, Kashiwagi A (1999) Effect of glycemic control on plasma plant sterol levels and post-heparin diamine oxidase activity in type 1 diabetic patients. Atherosclerosis 145:389–397
Sudhop T, Gottwald BM, von Bergman K (2002) Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism 51:1519–1521
Acknowledgements
This study was supported by grant 2001B043 from the Netherlands Heart Foundation (to A.K.G. and F.K.) and grant 902-23-262 from the Netherlands Organization for Scientific Research. We thank R. Havinga, R. Boverhof, J. Baller and C. van der Ley for their excellent technical assistance, Dr. F.G. Schaap for antibodies and Prof. Dr. P.J.J. Sauer for critical reading of the manuscript and his valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bloks, V.W., Bakker-van Waarde, W.M., Verkade, H.J. et al. Down-regulation of hepatic and intestinal Abcg5 and Abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia 47, 104–112 (2004). https://doi.org/10.1007/s00125-003-1261-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1261-y