Abstract
Key message
Two causal OsTTL and OsSAPK1 genes of the key locus qNL3.1 significantly associated with seed germination under salt stress were identified via a genome-wide association study, which could improve rice seed germination under salt stress.
Abstract
Rice is a salt-sensitive crop, and its seed germination determines subsequent seedling establishment and yields. In this study, 168 accessions were investigated for the genetic control of seed germination under salt stress based on the germination rate (GR), germination index (GI), time at which 50% germination was achieved (T50) and mean level (ML). Extensive natural variation in seed germination was observed among accessions under salt stress. Correlation analysis showed significantly positive correlations among GR, GI and ML and a negative correlation with T50 during seed germination under salt stress. Forty-nine loci significantly associated with seed germination under salt stress were identified, and seven of these were identified in both years. By comparison, 16 loci were colocated with the previous QTLs, and the remaining 33 loci might be novel. qNL3.1, colocated with qLTG-3, was simultaneously identified with the four indices in two years and might be a key locus for seed germination under salt stress. Analysis of candidate genes showed that two genes, the similar to transthyretin-like protein OsTTL and the serine/threonine protein kinase OsSAPK1, were the causal genes of qNL3.1. Germination tests indicated that both Osttl and Ossapk1 mutants significantly reduced seed germination under salt stress compared to the wild type. Haplotype analysis showed that Hap.1 of OsTTL and Hap.1 of OsSAPK1 genes were excellent alleles, and their combination resulted in high seed germination under salt stress. Eight accessions with elite performance of seed germination under salt stress were identified, which could improve rice seed germination under salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the most significant abiotic stresses in plants. It is estimated that over 6% of the world’s total land area and 20% of irrigated land are affected by salt stress (Munns and Tester 2008). Rice (Oryza sativa L.) is an important food crop worldwide. The frequent occurrence of soil salinity has been identified as the most widespread soil problem in rice production. Rice is rated as a salt-sensitive crop (Ahmadi et al. 2011). Soil salinity inhibits seed germination and seedling establishment, hampers plant growth throughout the whole growth stage and ultimately reduces crop yield in fields. Seed germination is a vital phase in the plant life cycle that determines subsequent seedling establishment and crop yield (Rajjou et al. 2012). Moreover, the direct seeding of rice has become increasingly popular in many Asian countries due to the lower labor demand and the operational simplicity (Kumar and Ladha 2011; Liu et al. 2015). Therefore, the improvement in seed germination under salt stress becomes more important in rice breeding.
Seed germination under salt stress is a complex quantitative trait and is regulated by polygenes. In Arabidopsis thaliana, the zinc finger-containing glycine-rich RNA-binding protein atRZ-1a, has a negative impact on seed germination under salt stress (Kim et al. 2007). Mitochondrial thioredoxin-o (AtTrxo1), which is transcriptionally regulated by the basic leucine zipper AtbZIP9 and the zinc finger protein AtAZF2, affects seed germination under salt stress (Ortiz-Espín et al. 2017). Glutamate receptor homolog 3.4-mediated Ca2+ influx is involved in the regulation of seed germination under salt stress by modulating Na+ accumulation through the SOS (salt overly sensitive) pathway in Arabidopsis (Cheng et al. 2018). The Arabidopsis WD40 repeat-containing protein XIW1, which interacts with abscisic acid (ABA) insensitive 5 (ABI5) in the nucleus and maintains its stability, promotes salt inhibition of seed germination (Cai et al. 2020). The ABI4-RbohD (NADPH oxidase genes)/VTC2 (vitamin C defective 2) regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress (Luo et al. 2021). The Arabidopsis long hypocotyl 2 (HY2) gene acts as a positive regulator of NaCl signaling during seed germination (Piao et al. 2021). In rice, the main locus qSE3, which encodes the K+ transport gene OsHAK21, was reported to promote seed germination and seedling establishment under salt stress through ABA metabolism (He et al. 2019). The expression of the basic helix-loop-helix (bHLH) TF OsbHLH035 is induced by salinity, and its mutants show delayed seed germination, particularly under salt stress conditions (Chen et al. 2018). The APETALA2 (AP2)-type transcription factor (TF), SALT AND ABA RESPONSE ERF1 (OsSAE1), acts as a positive regulator of seed germination and salt tolerance in rice by repressing OsABI5 expression (Li et al. 2022a). The exploration of the key genes associated with seed germination under salt stress deserves further investigation.
Genome-wide association studies (GWASs) are an effective strategy that use single-nucleotide polymorphisms (SNPs) as molecular genetic markers to detect valuable natural variation in trait-associated loci as well as allelic variations in candidate genes affecting complex traits (Huang et al. 2010; Zhao et al. 2011). With advances in gene sequencing and technical methods, GWASs have been successfully applied to determine quantitative trait loci (QTLs) and candidate genes of seed germination in plants, such as rice (Huang et al. 2021; Li et al. 2021a), maize (Huang et al. 2013), soybean (Kan et al. 2015; Liu et al. 2020), sorghum (Upadhyaya et al. 2016) and barley (Thabet et al. 2018). Recently, some studies involved in seed germination under salt stress have also been reported, such as in rice (Yu et al. 2018; Cui et al. 2018), barley (Mwando et al. 2020), flax (Li et al. 2022b), wheat (Hasseb et al. 2022) and oilseed rape (Zhang et al. 2022). However, it is unfortunate that the key genes of seed germination under salt stress identified via GWAS are still limited in rice.
In this study, we evaluated seed germination under salt stress using GR, GI, T50 and ML based on 168 diverse accessions from Rice Diversity Panel 1 (RDP1) (Zhao et al. 2011) in 2015 and 2017. The high-resolution, open-access research platform, which included 700 K SNP data (McCouch et al. 2016), was used for GWAS to identify the loci associated with rice seed germination under salt stress. Two candidate genes, the similar to transthyretin-like protein OsTTL and the serine/threonine protein kinase OsSAPK1, of a key locus qNL3.1 were obtained. Our studies indicated that the OsTTL and OsSAPK1 genes both positively regulated seed germination under salt stress, and their combination of excellent alleles had high potential for improving seed germination under salt stress in rice. It is helpful to elucidate the molecular mechanism of seed germination under salt stress and to improve seed germination in the direct seeding of rice, particularly in saline-alkali soil.
Materials and methods
Plant materials and growth
A total of 413 rice accessions from the Rice Diversity Panel 1 (RDP1) identified by Zhao et al. (2011) were obtained from Dr. Jian Hua at Cornell University (Table S1). Of these, 168 accessions, including 91 JAPONICA accessions, 56 INDICA accessions and 21 ADMIX accessions, were selected for evaluation of seed germination under salt stress. The mean germination rate (MGR) at 5 days after imbibition (DAI) of 168 accessions was more than 95% under H2O conditions in both 2015 and 2017. Osttl mutants (Osttl-1, Osttl-2, and Osttl-3) and Ossapk1 mutants (Ossapk1-1 and Ossapk1-1) were generated in the japonica Nipponbare background using the CRISPR/Cas9 system (Xing et al. 2014). All accessions were grown at the Jiangpu Experimental Station of Nanjing Agricultural University, Nanjing, Jiangsu. Field management was performed following the local standard methods (Cheng et al. 2015). All seeds were harvested at the maturity stage and dried at 42 ℃ for 7 days to break seed dormancy.
Evaluation of seed germination
A total of 30 healthy grains of each accession were surface-sterilized with 0.5% sodium hypochlorite solution for 15 min and then rinsed three times with sterile distilled water. Seeds were imbibed in 9-cm Petri dishes with 40-mL quartz and 20 mL H2O solution in a growth chamber at 25 ± 1 ℃ with a 12 h/12 h light–dark cycle for 10 days. Salt treatment used 200 mM NaCl solution instead of H2O solution. Germination was considered visually by the emergence of the radicle through the hull by ≥ 2 mm, and seedling establishment was considered when the root length reached the seed length and the shoot length reached half of the seed length (Cheng et al. 2015). The germination rate (GR) was calculated after 10 days of imbibition. T50 was calculated using GERMINATOR software (Joosen et al. 2010). GI was calculated as GI = ∑ (Gt/t), where Gt is the number of germinated seeds on Day t (Wang et al. 2010). GR and GI were evenly divided into 10 levels from 1 to 10 and T50 from 10 to 1, respectively, according to Qiu et al. (2015). The germination mean level (ML) of each accession was calculated as \(\frac{{{\text{ML\_GR}} + {\text{ML\_GI}} + {\text{ML}}\_T50}}{3}\) to evaluate the integrative capacity of seed germination under salt stress in this study. ML_GR, ML_GI and ML_T50 represent the mean levels of GR, GI and T50, respectively. Three replications of each accession were performed.
Genome-wide association study
A high-density array of 700 K SNPs described by McCouch et al. (2016) was used in this study. SNPs with minor allele frequencies (MAFs) ≤ 5% and ≥ 25% missing ratios were filtered (Yano et al. 2016) using TASSEL 5.2.40 software (Bradbury et al. 2007). The final set of SNPs included 403,950 subsequently obtained for GWAS. Linear mixed-model association studies in All population, and two sub-populations, INDICA and JAPONICA, not in ADMIX group due to fewer accessions, were implemented using the Efficient Mixed-Model Association eXpedited (EMMAX) in Linux (Kang et al. 2010). The significance threshold for all indices was set to P ≤ 1.0e-5, indicated by a red horizontal line in the Manhattan plot at − log10P ≥ 5, according to Crowell et al. (2016). GWAS results of seed germination under salt stress were visualized in Manhattan and quantile‒quantile plots (Q-Q plots, Fig. S1) using the R package qqman. The clear peak signals with significant SNP clusters (at least three SNPs, any two significant SNPs within a 200 kb interval) were considered as one associated locus (Lv et al. 2016). The most significant (i.e., the highest − log10P) SNP in a cluster was considered to be the lead SNP. There was more noise in the Manhattan plots as P ≤ 1.0e-5 for six traits, including GR, T50 and ML in INDICA in 2015, GR and T50 in INDICA in 2017 and GR in JAPONICA in 2017. To reduce the risk of false-positive loci associated with six traits, a Bonferroni correction (Li et al. 2017) was applied. Those loci in which the P value thresholds of the lead SNP were higher than 1.24e-7 (0.05/403,950) were filtered. Finally, the identified loci, for which the physical interval of their lead SNPs was less than 100 kb, were integrated into a locus, named qNLs.
Candidate gene analysis of qNL3.1
The candidate genomic region of the key locus qNL3.1 was calculated according to ± 100 kb of the flanking lead SNPs, and a 301.042-kb genomic region was obtained refer to the intersection of two years. To identify the causal genes of the qNL3.1 associated with seed germination under salt stress, the candidate genes were predicted with the Nipponbare reference genome (Lv et al. 2016) according to the MSU Rice Genome Annotation Project Release 7 database (http://rice.plantbiology.msu.edu). According to Yano et al. (2016), all the SNPs in the qNL3.1 region were categorized into five groups as their functions using the RiceVarMap v2.0 database (http://ricevarmap.ncpgr.cn/). Group I included significant SNPs (− log10P ≥ 5) that caused amino acid exchange. Group II included significant SNPs that were located in the promoter region and 5′ noncoding sequence. Group III included significant SNPs that were located within a coding region but not predicted to change an amino acid, or an intron or a 3′ noncoding sequence. Group IV included significant SNPs that were located in the intergenic region. Group V included SNPs that were not significantly associated with seed germination.
Expression analysis of candidate genes
The expression levels of five candidate genes, including LOC_Os03g27320, LOC_Os03g27250, LOC_Os03g27280, LOC_Os03g27310 and LOC_Os03g27360, were predicted in shoots, roots and seedlings under salt stress by GENEVESTIGATOR. Seeds of Nipponbare were sampled at 0, 6, 12, 24, 36, 48, 60 and 72 h after imbibition under 200 mM NaCl and quickly frozen in liquid nitrogen. All samples were stored at -80 ℃ for RNA extraction.
Total RNA was isolated from approximately 80∼100 mg powder with a total RNA Kit (BioTeke, www.bioteke.com). The first-strand cDNA was synthesized with random oligonucleotides using the HiScript II reverse transcription kit (Vazyme Biotech, http://www.vazyme.com/) according to the manufacturer’s protocol. To measure the mRNA levels of genes, quantitative real-time PCR (RT‒qPCR) was conducted by a CFX96 Real-time System (BIO-RAD, USA) with SYBR Green Mix (Vazyme). The rice housekeeping gene OsActin (LOC_Os03g50885) was used as an internal control (Pei et al. 2020). The PCR conditions were as follows: 95 ℃ for 5 min; 36 cycles of 95 ℃ for 15 s, 58 ℃ for 30 s and 72 ℃ for 1 min; and 72 ℃ for 5 min. A final melt curve stage of 65–95 ℃ was performed to confirm the specificity of the primers. Relative quantification of the transcript levels was obtained based on the 2−ΔΔ CT method (Livak and Schmittgen 2001). The amount of the transcripts in the dry Nipponbare seeds (0 h) was set at 1.0. All of the primer pairs had amplification efficiencies of approximately 100% and were designed according to http://quantprime.mpimp-golm.mpg.de/ and used for RT‒qPCR (Table S2). Three biological replicates were conducted.
Generation and identification of transgenic plants
The CRISPR/Cas9 plasmid was designed according to a protocol described previously (Xing et al. 2014). Two target sites (target 1 and target 2) of OsTTL and OsSAPK1 were confirmed via CRISPR-PLANT (http://www.genome.arizona.edu/crispr/CRISPRsearch.html), respectively. The target 964-bp segments that carry five elements, including target 1, gRNA-Sc, OsU3t, TaU3p and target 2, were amplified from the pCBC-MT1T2 vector using OsTTL (OsSAPK1)- BsF, F0, R0 and BsR primers. Then, the target 964-bp segments were cloned into the pHUE411 vector and verified using TaU3-FD2, TaU3-RD and OsU3-FD3 primers. The pCBC-MT1T2 and pHUE411 vectors were obtained from Qijun Chen’s laboratory (College of Biological Sciences, China Agricultural University, China). Transgenic rice plants were generated using the Agrobacterium-mediated cocultivation method in the background of Nipponbare. Genomic DNA was extracted from mutant seedlings using the cetyltrimethylammonium bromide (CTAB) method (Murray and Thompson 1980). The DNA fragments surrounding the two target sites of OsTTL and OsSAPK1 were amplified as specific OsTTL-CRISPR-F/R and OsSAPK1-CRISPR-F/R primers, respectively, and directly sequenced to detect homozygous positive mutants. T2 plants of the CRISPR/Cas9 mutants were used for phenotype analysis. All the primers are listed in Table S2.
Haplotype and combination analyses of OsTTL and OsSAPK1
For the OsTTL and OsSAPK1 genes, the haplotypes were classified based on all SNPs with an MAF > 0.05 in a function range including the 5' flanking sequences of genes (≤ 2 kb from the first ATG) and the CDS of the target gene (Butardo et al. 2017). The haplotypes containing at least five investigated accessions were used for comparative analysis (Wang et al. 2015). According to the haplotypes of OsTTL and OsSAPK1, the phenotypes of all accessions combined were compared for excellent combinations of OsTTL and OsSAPK1 to improve seed germination under salt stress.
Identification of salt-tolerant accessions
The phenotypic values of the ML showed the integrative capacity of seed germination under salt stress. The 5% of accessions with the highest phenotypic values of seed germination under salt stress were selected based on the ML. The alleles of the causal genes OsTTL and OsSAPK1 of qNL3.1 were analyzed among the selected accessions, and the best cross combinations were predicted to improve rice seed germination under salt stress.
Data analysis
Phenotypic data and variance were calculated by Excel 2017 software. The significant differences were tested using Student’s t test or Fisher’s least significant difference (LSD) test at the 5% and 1% levels of probability. Broad-sense heritability among 168 accessions was calculated using the method described by Lu et al. (2015). The correlation coefficients for multiple indices related to seed germination were calculated using the R package corrplot.
Results
Characterization of seed germination under salt stress
The 168 accessions of rice were evaluated via four indices of seed germination, including GR, GI, T50 and ML, under salt stress in 2015 and 2017 (Table S1). Phenotypic statistics showed that the GR, GI, T50 and ML of this population under salt stress ranged from 24.00 to 100.00%, 1.04 to 13.13, 1.89 to 12.00, and 1.67 to 9.67, respectively. The average values of the GR, GI, T50 and ML in 2015 and 2017 were 93.76% and 93.84%, 7.54 and 6.96%, 3.62 and 4.20%, and 7.72 and 7.35%, respectively (Table 1). Approximately normal distributions were observed in GI, T50 and ML, and skewed distributions were observed in GR in the two years (Fig. S2a–d), revealing that there was extensive phenotypic variation in GR, GI, T50 and ML under salt stress among this population.
By comparison among the All, JAPONICA and INDICA subgroups, there were no significant differences in the mean values in GR, GI, T50 and ML during seed germination under salt stress (Table S1; Fig. S2 e–h). However, the CVs of GR, GI, T50 and ML in the INDICA subgroup were higher than those in the JAPONICA subgroup (Table 1), suggesting that the INDICA subgroup may have a relatively larger phenotypic variation than the JAPONICA subgroup. In addition, two-way analysis of variance (ANOVA) revealed that the broad-sense heritabilities of GR, GI, T50 and ML were more than 65% in 2015 and 2017, and the G × E interactions were all significant (P < 0.001) (Table 1). These results suggested that seed germination under salt stress is regulated by both genetic and environmental factors.
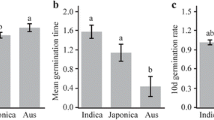
Furthermore, we calculated the correlation coefficients among the four indices of seed germination under salt stress over two years. There were significantly positive correlations among GR, GI and ML and negative correlations between T50 and the other three indices (Fig. 1). The highest correlation coefficients were found between ML and the other three indices, suggesting that ML might be a more effective index for the evaluation of seed germination under salt stress.
Loci associated with seed germination under salt stress
Based on four indices, GR, GI, T50 and ML, in the two years, we conducted GWAS for seed germination under salt stress using the Efficient Mixed-Model Association eXpedited (EMMAX) model according to Crowell et al. (2016). A total of 56 loci were significantly associated with the four indices for seed germination under salt stress, including 35 in 2015 and 21 in 2017 (Figs. 2 and S3–4; Table S3). Seven loci, including qNL1.8, qNL3.1, qNL3.2, qNL4.1, qNL7.2, qNL7.4 and qNL11.4, were simultaneously identified in 2015 and 2017 (Figs. 2 and 3); as a result, 49 loci in total were identified in two years in this study. Compared with the QTLs reported, 16 loci were colocalized with the reported QTLs, and the remaining 33 loci might be the novel loci identified in this study (Table S3).
Genome-wide association analysis of GR, GI, T50 and ML in INDICA. Manhattan plots of GR, GI, T50 and ML in 2015 (left) and 2017 (right). The horizontal red solid lines indicate a statistically significant threshold of P < 1 × 10–5. The horizontal red dotted lines indicate the Bonferroni-corrected threshold of P < 1 × 10–7. The arrows indicate the loci identified in both years (colour figure online)
Among the seven loci simultaneously identified in the two years, two loci, qNL3.1 and qNL3.2, were associated with all four indices of seed germination under salt stress, and three loci, qNL3.1, qNL3.2 and qNL4.1, were associated with at least three indices of seed germination (Fig. 3). In addition, seven loci, qNL1.10, qNL3.1, qNL5.4, qNL7.2, qNL7.4, qNL11.2 and qNL11.4, were identified in both the All and INDICA groups; two loci, qNL8.2 and qNL7.6, were identified in both the All and JAPONICA groups, and no colocalized loci were identified in both the JAPONICA and INDICA groups (Table S3).
One locus, qNL3.1, identified in both the All and INDICA groups was associated with the four indices in the two years (Figs. 2 and 3), indicating that it might be a key locus for rice seed germination under salt stress. We found that the key qNL3.1 was colocalized with qLTG-3 (Jiang et al. 2006), and its region contained the serine/threonine protein kinase OsSAPK1 (LOC_Os03g27280). Mutants of the OsSAPK1 gene were reported to exhibit lower germination rates under salt stress than wild-type plants (Lou et al. 2018). Therefore, we focused on qNL3.1 for further investigation.
Candidate genes of the key qNL3.1
To identify the causal gene of qNL3.1 controlling seed germination under salt stress, we analyzed all the candidate genes within a 301.042 kb region of qNL3.1 with the Nipponbare reference genome (http://rice.plantbiology.msu.edu) and identified 51 candidate genes after the removal of 16 genes encoding transposons and retrotransposons (Table S4). The classified results according to Yano et al. (2016) showed that only five genes were targeted and associated with the significant SNPs of qNL3.1 (Table S5). LOC_Os03g27320, located within Group I, had one significant SNP, SNP-3.15650453, which resulted in nonsynonymous variants on the CDS with amino acid residue changes from lysine (Lys) in accessions with “AA” to asparagine (Asn) in accessions with “TT” (Lys309Asn) (Fig. 4a; Table S5), indicating that it might be one of the causal genes of qNL3.1. The other four genes, LOC_Os03g27250, OsSAPK1 (LOC_Os03g27280), LOC_Os03g27310 and LOC_Os03g27360, were located within Group II. The significant SNPs SNP-3.15612217, SNP-3.15631187 and SNP-3.15645395 were located in the promoter regions of LOC_Os03g27250, OsSAPK1 and LOC_Os03g27310,, respectively, and SNP-3.15665224 and SNP-3.15665816 were located in the promoter regions of LOC_Os03g27360 (Fig. 4a; Table S5). The five SNPs were located on the cis-acting elements CGACC, RTTTTTR, CCGAC, AGAAA and GTAC. These results suggest that the expression levels of the four genes may be tightly associated with seed germination under salt stress.
Analysis and expression levels of candidate genes for the key locus qNL3.1. a Information on significant SNPs in candidate genes. The gray and blue bars indicate the promoter regions and CDSs of candidate genes, respectively. The red font indicates a variant base or amino acid. b Heatmap showing the differential expression of candidate genes of qNL3.1 based on the array database deposited in GENEVESTIGATOR. Red, upregulation; green, downregulation. c Expression patterns of OsTTL (LOC_Os03g27320) and OsSAPK1 (LOC_Os03g27280) under H2O and NaCl conditions. The relative expression levels were calibrated against the expression of the OsActin gene and quantified by RT‒qPCR. **Indicates a significant difference at the 0.01 level. ns indicates not statistically significant (colour figure online)
LOC_Os03g27320, LOC_Os03g27250, OsSAPK1, LOC_Os03g27310 and LOC_Os03g27360 encode a steroid-binding protein, OsFBO14, F-box and another domain containing protein, CAMK_CAMK_like.19. CAMK includes calcium/calmodulin dependent protein kinases, histone H3, and the RING-H2 finger protein ATL5H precursor (Table S5), respectively.
The expression profiles of the five candidate genes of qNL3.1 in shoots, roots and seedlings under salt stress were determined based on the array database deposited in GENEVESTIGATOR. The results showed that the expression levels of only OsSAPK1 in the shoots and seedlings were significantly upregulated under salt stress (Fig. 4b). Furthermore, we detected the expression of LOC_Os03g27320 and OsSAPK1 with the RT‒qPCR approach during seed germination under H2O and 200 mM NaCl conditions. The expression of LOC_Os03g27320 gradually decreased from 6 to 72 h after imbibition under H2O conditions, and the expression levels of OsSAPK1 increased from 0 to 12 h after imbibition and then decreased (Fig. 4c). Compared with that under H2O conditions, the expression levels of LOC_Os03g27320 were upregulated after imbibition for 6 h and from 36 to 72 h under 200 mM NaCl conditions, and the expression level of OsSAPK1 was dramatically upregulated during seed germination (Fig. 4c). Overall, LOC_Os03g27320 and OsSAPK1 were both induced by salt treatment and might be causal genes of qNL3.1.
Effect of seed germination of Osttl mutants under salt stress
LOC_Os03g27320 is described similar to the transthyretin-like (TTL) protein with 334 amino acids (Fig. S5), designated OsTTL. To characterize the function of OsTTL in seed germination under salt stress, we employed the CRISPR/Cas9 system to generate mutants of OsTTL with japonica Nipponbare and obtained three Osttl mutants, Osttl-1, Osttl-2 and Osttl-3 (Fig. 5a). Osttl-1 contained a 13-bp (GTGAACGGGAGCC) deletion in the first exon and an “A” insertion in the second exon of OsTTL; Osttl-2 contained a 14-bp (CGTGCTGCGCGTGA) deletion in the first exon and an “A” insertion in the second exon of OsTTL; and Osttl-3 contained a 24-bp (CCCGTCGAGGACGTGCTGCGCGTG) deletion in the first exon and an “A” deletion in the second exon of OsTTL (Fig. 5a). As predicted based on these nucleotide sequences, the amino acid sequence of OsTTL in Osttl-1 contains 330 amino acids, breaking the 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline decarboxylase (OHCU_decarbox) domain; the amino acid sequences of OsTTL in Osttl-2 and Osttl-3 contain 106 and 102 amino acids, respectively, due to the premature termination of OsTTL (Fig. S5). These results indicated that the three Osttl mutants might lack the function of OsTTL.
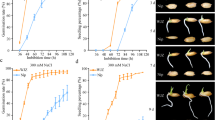
Seed germination is regulated by OsTTL under H2O and salt stress. a Generation of Osttl mutants via the CRISPR/Cas9 system. Green rectangles represent the exons of OsTTL. White rectangles represent the UTRs of OsTTL. Red lines and bases represent deletions or insertions in the genome of OsTTL. b Seed germination of Osttl mutants and WT after 4 and 6 days of imbibition under H2O and NaCl conditions. Comparison of GR, GI, T50 and ML between the Osttl mutants and WT under H2O (c) and NaCl (d) conditions. Bars = 5 cm. The red horizontal dotted lines indicate the mean value of WT. *, ** Indicates significant differences at the 0.05 and 0.01 levels, respectively. ns indicates not statistically significant (colour figure online)
The progeny of three homozygous Osttl mutants (T2) was selected, and their GR, GI, T50 and ML were evaluated during seed germination under H2O and 200 mM NaCl conditions. Under H2O conditions, the GR and ML values in Osttl-1, Osttl-2 and Osttl-3 were not significantly different from those in WT, but the GI significantly decreased and the T50 significantly increased (Fig. 5b–c). Under salt conditions, the GR, GI and ML values in Osttl-1, Osttl-2 and Osttl-3 were significantly decreased compared to those in WT, and the T50 significantly increased (Fig. 5b, d). In addition, the seedling rate (SR) was measured during seed germination under both H2O and 200 mM NaCl conditions. The results showed that the SR values in Osttl-1, Osttl-2 and Osttl-3 were significantly decreased compared to that in WT under both H2O and 200 mM NaCl conditions (Fig. S6). These results demonstrated that the Osttl mutants resulted in low germination speed and seedling growth during seed germination under salt stress, suggesting that OsTTL could positively regulate seed germination under salt stress.
Effect of seed germination of Ossapk1 mutants under salt stress
To confirm the function of OsSAPK1 in rice seed germination under salt stress, we employed the CRISPR/Cas9 system to generate mutants of OsSAPK1 with japonica Nipponbare and obtained two mutants, Ossapk1-1 and Ossapk1-2 (Fig. 6a). Ossapk1-1 contained an 81-bp (TCCGGGAACTTCGGGGTGGCCAAGCTCGTCCGCGACGTCGCCACCAACCACCTCTTCGCCGTCAAGTTCATCGAGAGGGGA) deletion in the first exon of OsSAPK1, and Ossapk1-2 contained a “T” deletion and a “C” insertion in the first exon of OsSAPK1 (Fig. 6a). The product of OsSAPK1 has 342 amino acids. As predicted based on these nucleotide sequences, the amino acid sequences of OsSAPK1 in Ossapk1-1 and Ossapk1-2 contained 315 and 342 amino acids, breaking their serine/threonine protein kinase domains (Fig. S7). These results indicated that the two Ossapk1 mutants might lack the function of OsSAPK1.
Seed germination is regulated by OsSAPK1 under H2O and salt stress. a Generation of Ossapk1 mutants via the CRISPR/Cas9 system. Green rectangles represent the exons of OsSAPK1. White rectangles represent the UTRs of OsSAPK1. Red lines and bases represent deletions or insertions in the genome of OsSAPK1. b Seed germination of Ossapk1 mutants and WT after 4 and 6 days of imbibition under H2O and NaCl conditions. Comparison of GR, GI, T50 and ML between the Ossapk1 mutants and WT under H2O (c) and NaCl (d) conditions. Bars = 5 cm. The red horizontal dotted lines indicate the mean value of WT. *, ** indicate significant differences at the 0.05 and 0.01 levels, respectively. ns indicates not statistically significant (colour figure online)
The progeny of two homozygous Ossapk1 mutants (T2) was selected, and their GR, GI, T50 and ML values were evaluated during seed germination under H2O and 200 mM NaCl conditions. Under H2O conditions, the GR and ML in Ossapk1-1 and Ossapk1-2 were not significantly different from those in WT, but the GI significantly decreased, and the T50 significantly increased (Fig. 6b–c). Under salt conditions, the GR, GI and ML in Ossapk1-1 and Ossapk1-2 were significantly decreased compared to those in WT, and the T50 significantly increased (Fig. 6b, d). Similarly, the SR was measured during seed germination under H2O and 200 mM NaCl conditions. The results showed that the SR in Ossapk1-1 and Ossapk1-2 were significantly decreased compared to that in WT under both H2O and 200 mM NaCl conditions (Fig. S8). These results demonstrated that the Ossapk1 mutants resulted in low seed germination and seedling growth during seed germination under both H2O and 200 mM NaCl conditions, suggesting that OsSAPK1 could positively regulate rice seed germination under salt stress.
Haplotypes and their combinations of OsTTL and OsSAPK1
Based on significant SNPs located on these two causal genes, 2 and 2 haplotypes were detected in the OsTTL and OsSAPK1 genes among the 168 accessions, respectively (Fig. 7a–b). For the OsTTL gene, the GR and ML of Hap.1 (C) were significantly higher than those of Hap.2 (T) under salt stress in both 2015 and 2017, and the T50 was significantly lower during seed germination (Fig. 7a). For the OsSAPK1 gene, the GR and ML of Hap.1 (A) were significantly higher than those of Hap.2 (T) under salt stress in both 2015 and 2017 (Fig. 7b), and the T50 was significantly lower during seed germination (Fig. 7b). These results suggest that both Hap.1 of OsTTL and Hap.1 of OsSAPK1 could result in higher seed germination under salt stress.
Plots of GR, GI, T50 and ML of accessions containing different haplotypes in 2015 and 2017. a Plots of GR, GI, T50 and ML of accessions containing the Hap.1 (C) and Hap.2 (T) haplotypes of OsTTL. b Plots of GR, GI, T50 and ML of accessions containing the Hap.1 (A) and Hap.2 (T) haplotypes of OsSAPK1. Haplotype combinations of OsTTL and OsSAPK1 in 2015 (c) and 2017 (d). ***Indicates a significant difference at the 0.001 level. Different lowercase letters indicate significant differences at the 0.05 level
Meanwhile, the combination analysis of the OsTTL and OsSAPK1 haplotypes showed that the combination (CA) of the excellent Hap.1 (C) of OsTTL and Hap.1 (A) of OsSAPK1 was significantly higher than other haplotypic combinations (TA and TT) in terms of GR, GI and ML in both 2015 and 2017 (Fig. 7c–d) and significantly lower than that in T50 (Fig. 6c–d), suggesting that the combination of the excellent alleles of OsTTL and OsSAPK1 had the potential to improve seed germination under salt stress in rice.
Identification of salt-tolerant accessions during seed germination
Given the phenotype of seed germination of accessions under salt stress, the top eight elite accessions were identified from 168 accessions, including 5 accessions in the IND group, O-Luen-Cheung, Byakkoku Y 5006 Seln, Criollo La Fria, SLO 17 and Kiang-Chou-Chiu, and 3 accessions in the ADMIX group, Palmyra, Saturn and KPF-16 (Fig. 8). Interestingly, the six elite accessions all carried the excellent Hap.1 of OsTTL and Hap.1 of OsSAPK1 (Fig. 8), indicating that these accessions could be selected with improved seed germination under salt stress in rice breeding.
Discussion
Seed germination is a critical stage initiating the life cycle of a plant. In this study, to evaluate seed germination under salt stress, we selected 200 accessions with adjacent heading dates (growth duration) grown in Nanjing from 413 accessions (RDP1, Zhao et al. 2011). To further decrease the environmental effect, we deleted another 32 accessions with abnormal germination rates under normal conditions in this study, and 168 were subjected to the experiment for two years. These accessions had extensive phenotypic variation in seed germination under salt stress in GR, GI, T50 and ML, suggesting that they might be suitable for a GWAS. Moreover, INDICA exhibited larger phenotypic variation than JAPONICA, consistent with the results reported by Yang et al. (2022), indicating that there are likely some key genes regulating seed germination under salt stress in INDICA. Previous studies showed that INDICA accessions presented stronger seed vigor during seed germination than JAPONICA (Wang et al. 2010), while JAPONICA was more tolerant to cold and salt during seed germination than INDICA (Pan et al. 2015; Islam et al. 2022). No significant differences were observed in seed germination under salt stress between INDICA and JAPONICA in our study. This is different from the results under salt stress reported by Islam et al. (2022), possibly due to the differences in their populations and salt concentrations.
The identification of QTLs controlling seed germination under salt stress would contribute to understanding the genetic control and elucidating the processes of seed germination under salt stress. In this study, we identified 49 loci significantly associated with seed germination under salt stress via GWAS in 2015 and 2017 and found that seven loci, qNL1.8, qNL3.1, qNL3.2, qNL4.1, qNL7.2, qNL7.4 and qNL11.4, were identified in both years, suggesting that their genetic effects were relatively stable in rice fields. These stable loci are worthy of further exploration for the novel genes regulating seed germination under salt stress, which would aid in understanding the genetic control and elucidating the processes of seed germination under salt stress in rice. The other 42 loci were not identified in both years, which may be due to different states of grain development because of the environmental conditions. Comparing chromosomal locations of reported QTLs, there were approximately one-third of loci colocated with the reported QTLs. For example, qNL6.3 was close to the position of qRI6 (Islam et al. 2022), including the OsSAE1 gene (Li et al. 2022b), which regulates seed germination under salt stress. qNL1.1 and qNL1.6 were colocated with the positions of OsNLP3 (Yi et al. 2022) and OsMYB3R-2 (Dai et al. 2007) regulating seed germination under salt stress, respectively. qNL11.3 was colocated with the position of Rab16A (Ganguly et al. 2012), enhancing salt tolerance at the seedling stage. This finding suggests that the identified loci of seed germination under salt stress in our study with GR, GI, T50 and ML by GWAS are reliable.
It is well known that the selection of effective indices is important for evaluating targeted traits correctly. In previous studies, Wang et al. (2010) evaluated the phenotype of seed germination under H2O and salt conditions using the imbibition rate and germination percentage and identified 16 QTLs of rice seed germination via the recombinant inbred lines (RILs, F2:9) population derived from a cross between japonica Jiucaiqing and indica IR26. Zeng et al. (2021) also assessed the phenotype of seed germination under H2O and salt conditions using GR and GI and identified 13 QTLs for seed germination via the BC1F2 population derived from the crossing Wujiaozhan/Nip (Nipponbare)//Nip. Lai et al. (2016) determined the characteristics of seed vigor using the germination potential, GR, GI and T50 and identified 19 additive and 2 epistatic quantitative trait loci for seed vigor via RIL populations. Shi et al. (2017) analyzed salt tolerance at the seed germination stage using the stress susceptibility indices of vigor index and mean germination time based on 478 rice accessions and identified eleven significantly associated loci via GWAS. This shows that seed germination is a complicated trait that can be evaluated using several indices, e.g., GR, GI and T50. However, there are few comprehensively evaluated indices used. In our study, we divided GR, GI and T50 into 10 levels according to Qiu et al. (2015) and set the ML index through the integrated GR, GI and T50 indices. Our results showed that 42, 4, 27 and 23 loci were significantly associated with GR, GI, T50 and ML, respectively, suggesting that the number of identified loci with ML is relatively appropriate. Meanwhile, of 23 loci identified by ML, 18, 4 and 18 loci colocalized with GR, GI and T50, respectively, and significant and tight correlations of ML with the GR, GI and T50 indices were observed, implying that ML, as a comprehensive index, is effective for the evaluation of seed germination.
In Arabidopsis thaliana, the protein TTL is a potential substrate of BR-INSENSITIVE-1 (BRI1) and is involved in brassinosteroid (BR)-mediated plant growth (Nam et al. 2004). BRs, as a class of polyhydroxylated steroid plant hormones, participate in the regulation of seed germination (Steber and McCourt 2001; Bajguz et al. 2020) as well as in responding to environmental stresses (Soares et al. 2020; Kim et al. 2019). In rice, Xiong et al. (2022) reported that BRs could regulate rice seed germination through the BZR1 (brassinazole-resistant 1)-RAmy3D (alpha-amylase 3D) transcriptional module. TTL acts as a bifunctional enzyme, 5-hydroxyisocyanate (5-HIU) hydrolase and 2-oxo-4-hydroxy-4-carboxy-5-ureidoimidazoline decarboxylase (OHCU_decarbox), which catalyze two steps in the allantoin biosynthesis pathway (Lamberto et al. 2010; Pessoa et al. 2010). In addition, Liu et al. (2020) reported that allantoin might be a key regulator of sugar beet salt tolerance. As predicted by https://www.uniprot.org/, the OsTTL protein contained OHCU_decarbox and transthyretin (TR_THY, Fig. S5), and seed germination and seedling growth in Osttl mutants were significantly reduced under salt stress. These findings suggest that OsTTL plays important roles in seed germination under salt stress in rice and that the salt tolerance mechanism may be involved in the BR and allantoin biosynthesis pathways.
It is well known that SnRK2s (sucrose nonfermenting1-related protein Kinase 2), as core components of the ABA signaling pathway, bind to and phosphorylate AREB/ABF (ABA responsive element-binding protein/ABRE-binding factor) transcription factors (Liu et al. 2019) to participate in various biological processes, including seed germination and salt tolerance (Li et al. 2021b; Wang et al. 2020). Rice contains 10 SnRK2 members denoted as SAPK1-10 (stress-activated protein kinase) (Kobayashi et al. 2004; Liu et al. 2019), and Ossapk1 mutants have been reported to exhibit reduced seed germination under salt stress (Lou et al. 2018). This result is consistent with the results that the seed germination and seedling growth in Ossapk1 mutants were significantly reduced under salt stress compared to WT in our study.
In our study, qNL3.1, which was simultaneously identified by the four indices of seed germination under salt stress over the two years, is a key locus for seed germination under salt stress. Based on the classification of significant SNPs of qNL3.1 according to Yano et al. (2016) and the expression levels of candidate genes, we hypothesized that OsTTL and OsSAPK1 are both causal genes of qNL3.1 that regulate rice seed germination under salt stress. However, since the genotype set for GWAS could not cover all the SNPs, it is possible that there are still other genes, whose SNPs are not included in the GWAS set, to regulate seed germination within the region of qNL3.1. To date, there are no reports that two or multiple genes have been identified to regulate seed germination at a locus, such as disease resistance-related genes (Deng et al. 2017; Chen et al. 2015). Previous reports showed that allantoin could activate the production of ABA, enhancing abiotic stress tolerance in plants (Watanabe et al. 2014; Takagi et al. 2016). OsSAPK1, an ABA-activated protein kinase (Lou et al. 2018), might be involved in ABA and its signaling pathways. It seems that there is crosstalk between OsTTL and OsSAPK1 that regulates seed germination under salt stress. The regulatory mechanisms of OsTTL and OsSAPK1 and their relationship or crosstalk in seed germination under salt stress should be studied in the future.
The genotypic selection strategy is superior to phenotypic selection in accelerating gene pyramiding by MAS (Xu et al. 2012). The optimal breeding scheme of gene pyramiding involves selecting a series of favorite target alleles after crossing base populations and pyramiding them into a single genotype (Servin et al. 2004; Xu et al. 2012). Among haplotype groups, the excellent Hap.1 of OsTTL and Hap.1 of OsSAPK1 have higher contributions to seed germination under salt stress, which needs further confirmation via a transgenic approach. The combination of the excellent Hap.1 of OsTTL and Hap.1 of OsSAPK1 could be used for gene pyramiding to develop salt-tolerant rice varieties with higher seed germination ability under salt stress. Moreover, six elite accessions, O-Luen-Cheung, Byakkoku Y 5006 Seln, Saturn, SLO 17, KPF-16 and Kiang-Chou-Chiu, carrying the combination of Hap.1 of OsTTL and Hap.1 of OsSAPK1, with high seed germination under salt stress in rice, would be useful in future rice breeding programs. Moreover, the physical positions between the OsTTL and OsSAPK1 genes, both located within qNL3.1, were close, within approximately 19 kb on chromosome 3. Thereby, the excellent alleles of OsTTL and OsSAPK1 genes, as a combination, could be quickly pyramided with other cloned excellent genes into one cultivar with reduced rounds of crossing in rice breeding.
Conclusion
In this study, we identified a total of 60 loci significantly associated with seed germination under salt stress via a genome-wide association study, including ten loci identified in two years. Of these loci, the key locus qNL3.1 was simultaneously identified with the four indices in the two years, and its two causal genes, OsTTL and OsSAPK1, positively regulated rice seed germination under stress. The excellent Hap.1 of OsTTL and Hap.1 of OsSAPK1 were also found, and their combination could result in high seed germination under salt stress. Six accessions carrying the combination of Hap.1 of OsTTL and Hap.1 of OsSAPK1 were identified with elite performance of seed germination under salt stress. Our identified loci and elite accessions might be applicable to improve rice seed germination under salt stress in the future.
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online.
References
Ahmadi N, Negrão S, Katsantonis D, Frouin J, Ploux J, Letourmy P, Droc G, Babo P, Trindade H, Bruschi G, Greco R, Oliveira MM, Piffanelli P, Courtois B (2011) Targeted association analysis identified japonica rice varieties achieving Na+/K+ homeostasis without the allelic make-up of the salt tolerant indica variety Nona Bokra. Theor Appl Genet 123(6):881–895. https://doi.org/10.1007/s00122-011-1634-4
Bajguz A, Chmur M, Gruszka D (2020) Comprehensive overview of the brassinosteroid biosynthesis pathways: substrates, products, inhibitors, and connections. Front Plant Sci 11:1034. https://doi.org/10.3389/fpls.2020.01034
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23(19):2633–2635. https://doi.org/10.1093/bioinformatics/btm308
Butardo VM Jr, Anacleto R, Parween S, Samson I, de Guzman K, Alhambra CM, Misra G, Sreenivasulu N (2017) Systems genetics identifies a novel regulatory domain of amylose synthesis. Plant Physiol. https://doi.org/10.1104/pp.16.01248
Cai J, Huang H, Xu X, Zhu G (2020) An Arabidopsis WD40 repeat-containing protein XIW1 promotes salt inhibition of seed germination. Plant Signal Behav 15(2):1712542. https://doi.org/10.1080/15592324.2020.1712542
Chen HC, Cheng WH, Hong CY, Chang YS, Chang MC (2018) The transcription factor OsbHLH035 mediates seed germination and enables seedling recovery from salt stress through ABA-dependent and ABA-independent pathways, respectively. Rice (N Y) 11(1):50. https://doi.org/10.1186/s12284-018-0244-z
Chen J, Peng P, Tian J, He Y, Zhang L, Liu Z, Yin D, Zhang Z (2015) Pike, a rice blast resistance allele consisting of two adjacent NBS-LRR genes, was identified as a novel allele at the Pik locus. Mol Breed 35:117. https://doi.org/10.1007/s11032-015-0305-6
Cheng Y, Zhang X, Sun T, Tian Q, Zhang WH (2018) Glutamate receptor homolog3.4 is involved in regulation of seed germination under salt stress in Arabidopsis. Plant Cell Physiol 59(5):978–988. https://doi.org/10.1093/pcp/pcy034
Cheng J, He Y, Yang B, Lai Y, Wang Z, Zhang H (2015) Association mapping of seed germination and seedling growth at three conditions in indica rice (Oryza sativa L.). Euphytica 206:103–115. https://doi.org/10.1007/s10681-015-1477-1
Cui Y, Zhang F, Zhou Y (2018) The application of multi-locus GWAS for the detection of salt-tolerance loci in rice. Front Plant Sci 9:1464. https://doi.org/10.3389/fpls.2018.01464
Crowell S, Korniliev P, Falcão A, Ismail A, Gregorio G, Mezey J, McCouch S (2016) Genome-wide association and high-resolution phenotyping link Oryza sativa panicle traits to numerous trait-specific QTL clusters. Nat Commun 7:10527. https://doi.org/10.1038/ncomms10527
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K (2007) Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol 143(4):1739–1751. https://doi.org/10.1104/pp.106.094532
Deng Y, Zhai K, Xie Z, Yang D, Zhu X, Liu J, Wang X, Qin P, Yang Y, Zhang G, Li Q, Zhang J, Wu S, Milazzo J, Mao B, Wang E, Xie H, Tharreau D, He Z (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 355(6328):962–965. https://doi.org/10.1126/science.aai8898
Ganguly M, Datta K, Roychoudhury A, Gayen D, Sengupta DN, Datta SK (2012) Overexpression of Rab16A gene in indica rice variety for generating enhanced salt tolerance. Plant Signal Behav 7(4):502–509. https://doi.org/10.4161/psb.19646
Hasseb NM, Sallam A, Karam MA, Gao L, Wang RRC, Moursi YS (2022) High-LD SNP markers exhibiting pleiotropic effects on salt tolerance at germination and seedlings stages in spring wheat. Plant Mol Biol 108(6):585–603. https://doi.org/10.1007/s11103-022-01248-x
He Y, Yang B, He Y, Zhan C, Cheng Y, Zhang J, Zhang H, Cheng J, Wang Z (2019) A quantitative trait locus, qSE3, promotes seed germination and seedling establishment under salinity stress in rice. Plant J 97(6):1089–1104. https://doi.org/10.1111/tpj.14181
Huang J, Zhang J, Li W, Hu W, Duan L, Feng Y, Qiu F, Yue B (2013) Genome-wide association analysis of ten chilling tolerance indices at the germination and seedling stages in maize. J Integr Plant Biol 55(8):735–744. https://doi.org/10.1111/jipb.12051
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Li W, Lin Z, Buckler ES, Qian Q, Zhang QF, Li J, Han B (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42(11):961–967. https://doi.org/10.1038/ng.695
Huang Z, Ying J, Peng L, Sun S, Huang C, Li C, Wang Z, He Y (2021) A genome-wide association study reveals that the cytochrome b5 involved in seed reserve mobilization during seed germination in rice. Theor Appl Genet 134(12):4067–4076. https://doi.org/10.1007/s00122-021-03948-2
Islam MR, Naveed SA, Zhang Y, Li Z, Zhao X, Fiaz S, Zhang F, Wu Z, Hu Z, Fu B, Shi Y, Shah SM, Xu J, Wang W (2022) Identification of candidate genes for salinity and anaerobic tolerance at the germination stage in rice by genome-wide association analyses. Front Genet 13:822516. https://doi.org/10.3389/fgene.2022.822516
Joosen RV, Kodde J, Willems LA, Ligterink W, van der Plas LH, Hilhorst HW (2010) GERMINATOR: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J 62(1):148–159. https://doi.org/10.1111/j.1365-313X.2009.04116.x
Jiang L, Liu S, Hou M, Tang J, Chen L, Zhai H, Wan J (2006) Analysis of QTLs for seed low temperature germinability and anoxia germinability in rice (Oryza sativa L.). Field Crop Res 98:68–75. https://doi.org/10.1016/j.fcr.2005.12.015
Kan G, Zhang W, Yang W, Ma D, Zhang D, Hao D, Hu Z, Yu D (2015) Association mapping of soybean seed germination under salt stress. Mol Genet Genomics 290(6):2147–2162. https://doi.org/10.1007/s00438-015-1066-y
Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, Sabatti C, Eskin E (2010) Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42(4):348-54. https://doi.org/10.1038/ng.548
Kim SY, Warpeha KM, Huber SC (2019) The brassinosteroid receptor kinase, BRI1, plays a role in seed germination and the release of dormancy by cold stratification. J Plant Physiol 241:153031. https://doi.org/10.1016/j.jplph.2019.153031
Kim YO, Pan S, Jung CH, Kang H (2007) A zinc finger-containing glycine-rich RNA-binding protein, atRZ-1a, has a negative impact on seed germination and seedling growth of Arabidopsis thaliana under salt or drought stress conditions. Plant Cell Physiol 48(8):1170–1181. https://doi.org/10.1093/pcp/pcm087
Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16(5):1163–1177. https://doi.org/10.1105/tpc.019943
Kumar V, Ladha JK (2011) Direct seeding of rice. In: Advances in agronomy. pp 297–413. doi:https://doi.org/10.1016/b978-0-12-387689-8.00001-1
Lamberto I, Percudani R, Gatti R, Folli C, Petrucco S (2010) Conserved alternative splicing of Arabidopsis transthyretin-like determines protein localization and S-allantoin synthesis in peroxisomes. Plant Cell 22(5):1564–1574. https://doi.org/10.1105/tpc.109.070102
Lai Y, Cheng J, He Y, Yang B, Wang Z, Zhang H (2016) Identification of QTLs with additive, epistatic, and QTL × seed maturity interaction effects for seed vigor in rice. Plant Mol Biol Rep 34:160–171. https://doi.org/10.1007/s11105-015-0913-7
Li W, Yang B, Xu J, Peng L, Sun S, Huang Z, Jiang X, He Y, Wang Z (2021a) A genome-wide association study reveals that the 2-oxoglutarate/malate translocator mediates seed vigor in rice. Plant J 108(2):478–491. https://doi.org/10.1111/tpj.15455
Li X, Yu B, Wu Q, Min Q, Zeng R, Xie Z, Huang J (2021) OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice. PLoS Genet 17(8):e1009699. https://doi.org/10.1371/journal.pgen.1009699
Li X, Guo D, Xue M, Li G, Yan Q, Jiang H, Liu H, Chen J, Gao Y, Duan L, Xie L (2022) Genome-wide association study of salt tolerance at the seed germination stage in Flax (Linum usitatissimum L.). Genes. https://doi.org/10.3390/genes13030486
Li X, Guo Z, Lv Y, Cen X, Ding X, Wu H, Li X, Huang J, Xiong L (2017) Genetic control of the root system in rice under normal and drought stress conditions by genome-wide association study. PLoS Genet 13:e1006889. https://doi.org/10.1371/journal.pgen.1006889
Li Y, Zhou J, Li Z, Qiao J, Quan R, Wang J, Huang R, Qin H (2022b) SALT AND ABA RESPONSE ERF1 improves seed germination and salt tolerance by repressing ABA signaling in rice. Plant Physiol. https://doi.org/10.1093/plphys/kiac125
Liu H, Hussain S, Zheng M, Peng S, Huang J, Cui K, Nie L (2015) Dry direct-seeded rice as an alternative to transplanted-flooded rice in central China. Agron Sustain Dev 35(1):285–294. https://doi.org/10.1007/s13593-014-0239-0
Liu X, Li Z, Hou Y, Wang Y, Wang H, Tong X, Ao H, Zhang J (2019) Protein interactomic analysis of SAPKs and ABA-Inducible bZIPs revealed key roles of SAPK10 in rice flowering. Int J Mol Sci. https://doi.org/10.3390/ijms20061427
Liu Z, Li H, Gou Z, Zhang Y, Wang X, Ren H, Wen Z, Kang BK, Li Y, Yu L, Gao H, Wang D, Qi X, Qiu L (2020) Genome-wide association study of soybean seed germination under drought stress. Mol Genet Genomics 295(3):661–673. https://doi.org/10.1007/s00438-020-01646-0
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lou D, Wang H, Yu D (2018) The sucrose non-fermenting-1-related protein kinases SAPK1 and SAPK2 function collaboratively as positive regulators of salt stress tolerance in rice. BMC Plant Biol 18(1):203. https://doi.org/10.1186/s12870-018-1408-0
Lu Q, Zhang M, Niu X, Wang S, Xu Q, Feng Y, Wang C, Deng H, Yuan X, Yu H, Wang Y, Wei X (2015) Genetic variation and association mapping for 12 agronomic traits in indica rice. BMC Genomics 16:1067. https://doi.org/10.1186/s12864-015-2245-2
Luo X, Dai Y, Zheng C, Yang Y, Chen W, Wang Q, Chandrasekaran U, Du J, Liu W, Shu K (2021) The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol 229(2):950–962. https://doi.org/10.1111/nph.16921
Lv Y, Guo Z, Li X, Ye H, Li X, Xiong L (2016) New insights into the genetic basis of natural chilling and cold shock tolerance in rice by genome-wide association analysis. Plant Cell Environ 39(3):556–570. https://doi.org/10.1111/pce.12635
McCouch SR, Wright MH, Tung CW, Maron LG, McNally KL, Fitzgerald M, Singh N, DeClerck G, Agosto-Perez F, Korniliev P, Greenberg AJ, Naredo ME, Mercado SM, Harrington SE, Shi Y, Branchini DA, Kuser-Falcão PR, Leung H, Ebana K, Yano M, Eizenga G, McClung A, Mezey J (2016) Open access resources for genome-wide association mapping in rice. Nat Commun 7:10532. https://doi.org/10.1038/ncomms10532
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325. https://doi.org/10.1093/nar/8.19.4321
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Mwando E, Han Y, Angessa TT, Zhou G, Hill CB, Zhang XQ, Li C (2020) Genome-wide association study of salinity tolerance during germination in Barley (Hordeum vulgare L.). Front Plant Sci 11:118. https://doi.org/10.3389/fpls.2020.00118
Nam KH, Li J (2004) The Arabidopsis transthyretin-like protein is a potential substrate of BRASSINOSTEROID-INSENSITIVE 1. Plant Cell 16(9):2406–2417. https://doi.org/10.1105/tpc.104.023903
Ortiz-Espín A, Iglesias-Fernández R, Calderón A, Carbonero P, Sevilla F, Jiménez A (2017) Mitochondrial AtTrxo1 is transcriptionally regulated by AtbZIP9 and AtAZF2 and affects seed germination under saline conditions. J Exp Bot 68(5):1025–1038. https://doi.org/10.1093/jxb/erx012
Pan Y, Zhang H, Zhang D, Li J, Xiong H, Yu J, Li J, Rashid MA, Li G, Ma X, Cao G, Han L, Li Z (2015) Genetic analysis of cold tolerance at the germination and booting stages in rice by association mapping. PLoS One 10(3):e0120590. https://doi.org/10.1371/journal.pone.0120590
Pessoa J, Sárkány Z, Ferreira-da-Silva F, Martins S, Almeida MR, Li J, Damas AM (2010) Functional characterization of Arabidopsis thaliana transthyretin-like protein. BMC Plant Biol 10:30. https://doi.org/10.1186/1471-2229-10-30
Pei W, Jain A, Zhao B, Feng B, Xu D, Wang X (2020) Knockdown of OsSAE1a affects the growth and development and phosphate homeostasis in rice. J Plant Physiol 255:153275. https://doi.org/10.1016/j.jplph.2020.153275
Piao M, Zou J, Li Z, Zhang J, Yang L, Yao N, Li Y, Li Y, Tang H, Zhang L, Yang D, Yang Z, Du X, Zuo Z (2021) The Arabidopsis HY2 gene acts as a positive regulator of NaCl signaling during seed germination. Int J Mol Sci. https://doi.org/10.3390/ijms22169009
Qiu X, Yuan Z, Liu H, Xiang X, Yang L, He W, Du B, Ye G, Xu J, Xing D (2015) Identification of salt tolerance-improving quantitative trait loci alleles from a salt-susceptible rice breeding line by introgression breeding. Plant Breed 134:653–660
Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D (2012) Seed germination and vigor. Annu Rev Plant Biol 63:507–533. https://doi.org/10.1146/annurev-arplant-042811-105550
Servin B, Martin OC, Mézard M, Hospital F (2004) Toward a theory of marker-assisted gene pyramiding. Genetics 168(1):513–523. https://doi.org/10.1534/genetics.103.023358
Shi Y, Gao L, Wu Z, Zhang X, Wang M, Zhang C, Zhang F, Zhou Y, Li Z (2017) Genome-wide association study of salt tolerance at the seed germination stage in rice. BMC Plant Biol 17(1):92. https://doi.org/10.1186/s12870-017-1044-0
Soares T, Dias D, Oliveira AMS, Ribeiro DM, Dias L (2020) Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol Environ Saf 193:110296. https://doi.org/10.1016/j.ecoenv.2020.110296
Steber CM, McCourt P (2001) A role for brassinosteroids in germination in Arabidopsis. Plant Physiol 125(2):763–769. https://doi.org/10.1104/pp.125.2.763
Takagi H, Ishiga Y, Watanabe S, Konishi T, Egusa M, Akiyoshi N, Matsuura T, Mori IC, Hirayama T, Kaminaka H, Shimada H, Sakamoto A (2016) Allantoin, a stress-related purine metabolite, can activate jasmonate signaling in a MYC2-regulated and abscisic acid-dependent manner. J Exp Bot 67(8):2519–2532. https://doi.org/10.1093/jxb/erw071
Thabet SG, Moursi YS, Karam MA, Graner A, Alqudah AM (2018) Genetic basis of drought tolerance during seed germination in barley. PLoS One 13(11):e0206682. https://doi.org/10.1371/journal.pone.0206682
Upadhyaya HD, Wang YH, Sastry DV, Dwivedi SL, Prasad PV, Burrell AM, Klein RR, Morris GP, Klein PE (2016) Association mapping of germinability and seedling vigor in sorghum under controlled low-temperature conditions. Genome 59(2):137–145. https://doi.org/10.1139/gen-2015-0122
Wang L, Lu Q, Wen X, Lu C (2015) Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiol 169(4):2848–2862. https://doi.org/10.1104/pp.15.01170
Wang Y, Hou Y, Qiu J, Wang H, Wang S, Tang L, Tong X, Zhang J (2020) Abscisic acid promotes jasmonic acid biosynthesis via a 'SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol 228(4):1336–1353. https://doi.org/10.1111/nph.16774
Wang Z, Wang J, Bao Y, Wang F, Zhang H (2010) Quantitative trait loci analysis for rice seed vigor during the germination stage. J Zhejiang Univ Sci B 11(12):958–964. https://doi.org/10.1631/jzus.B1000238
Watanabe S, Matsumoto M, Hakomori Y, Takagi H, Shimada H, Sakamoto A (2014) The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ 37(4):1022–1036. https://doi.org/10.1111/pce.12218
Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14:327. https://doi.org/10.1186/s12870-014-0327-y
Xiong M, Yu J, Wang J, Gao Q, Huang L, Chen C, Zhang C, Fan X, Zhao D, Liu QQ, Li QF (2022) Brassinosteroids regulate rice seed germination through the BZR1-RAmy3D transcriptional module. Plant Physiol. https://doi.org/10.1093/plphys/kiac043
Xu LY, Zhao FP, Sheng XH, Ren HX, Zhang L, Wei CH, Du LX (2012) Optimal design for marker-assisted gene pyramiding in cross population. Asian Australas J Anim Sci 25(6):772–784. https://doi.org/10.5713/ajas.2011.11239
Yang B, Chen M, Zhan C, Liu K, Cheng Y, Xie T, Zhu P, He Y, Zeng P, Tang H, Tsugama D, Chen S, Zhang H, Cheng J (2022) Identification of OsPK5 involved in rice glycolytic metabolism and GA/ABA balance for improving seed germination via GWAS. J Exp Bot. https://doi.org/10.1093/jxb/erac071
Yano K, Yamamoto E, Aya K, Takeuchi H, Lo PC, Hu L, Yamasaki M, Yoshida S, Kitano H, Hirano K, Matsuoka M (2016) Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat Genet 48(8):927–934. https://doi.org/10.1038/ng.3596
Yi Y, Peng Y, Song T, Lu S, Teng Z, Zheng Q, Zhao F, Meng S, Liu B, Peng Y, Chen G, Zhang J, Ye N (2022) NLP2-NR module associated NO is involved in regulating seed germination in rice under salt stress. Plants (Basel). https://doi.org/10.3390/plants11060795
Yu J, Zhao W, Tong W, He Q, Yoon MY, Li FP, Choi B, Heo EB, Kim KW, Park YJ (2018) A genome-wide association study reveals candidate genes related to salt tolerance in rice (Oryza sativa) at the germination stage. Int J Mol Sci. https://doi.org/10.3390/ijms19103145
Zeng P, Zhu P, Qian L, Qian X, Mi Y, Lin Z, Dong S, Aronsson H, Zhang H, Cheng J (2021) Identification and fine mapping of qGR6.2, a novel locus controlling rice seed germination under salt stress. BMC Plant Biol 21(1):36. https://doi.org/10.1186/s12870-020-02820-7
Zhang G, Zhou J, Peng Y, Tan Z, Li L, Yu L, Jin C, Fang S, Lu S, Guo L, Yao X (2022) Genome-wide association studies of salt tolerance at seed germination and seedling stages in Brassica napus. Front Plant Sci 12:772708. https://doi.org/10.3389/fpls.2021.772708
Zhao K, Tung CW, Eizenga GC, Wright MH, Ali ML, Price AH, Norton GJ, Islam MR, Reynolds A, Mezey J, McClung AM, Bustamante CD, McCouch SR (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2:467. https://doi.org/10.1038/ncomms1467
Acknowledgements
The authors would like to thank Dr. Jian Hua at Cornell University and Nanjing Agricultural University for seeds and Dr. Susan McCouch at Cornell University for input on using this panel for GWAS.
Funding
This research was supported by Hainan Yazhou Bay Seed Laboratory (project of B21HJ1002), the National Natural Science Foundation of China (Grant No. 32272169, 32172037 and 32000377) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20201322).
Author information
Authors and Affiliations
Contributions
Z, HZ and JC designed the experiments. CZ and PZ carried out the main experimental work. YC, XC, KL, SC, JH, YH, TX, SL, ZY, SC and HT participated in part of the experimental work. CZ and JC analyzed the data and wrote the first draft of the manuscript. HZ and JC revised and implemented the final version of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Matthias Wissuwa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhan, C., Zhu, P., Chen, Y. et al. Identification of a key locus, qNL3.1, associated with seed germination under salt stress via a genome-wide association study in rice. Theor Appl Genet 136, 58 (2023). https://doi.org/10.1007/s00122-023-04252-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04252-x