Abstract
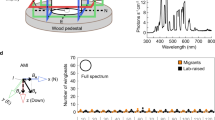
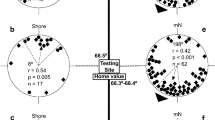
Here, we provide evidence for a wavelength-dependent effect of light on magnetic compass orientation in Pelophylax perezi (order Anura), similar to that observed in Rana catesbeiana (order Anura) and Notophthalmus viridescens (order Urodela), and confirm for the first time in an anuran amphibian that a 90° shift in the direction of magnetic compass orientation under long-wavelength light (≥500 nm) is due to a direct effect of light on the underlying magnetoreception mechanism. Although magnetic compass orientation in other animals (e.g., birds and some insects) has been shown to be influenced by the wavelength and/or intensity of light, these two amphibian orders are the only taxa for which there is direct evidence that the magnetic compass is light-dependent. The remarkable similarities in the light-dependent magnetic compasses of anurans and urodeles, which have evolved as separate clades for at least 250 million years, suggest that the light-dependent magnetoreception mechanism is likely to have evolved in the common ancestor of the Lissamphibia (Early Permian, ~294 million years) and, possibly, much earlier. Also, we discuss a number of similarities between the functional properties of the light-dependent magnetic compass in amphibians and blue light-dependent responses to magnetic stimuli in Drosophila melanogaster, which suggest that the wavelength-dependent 90° shift in amphibians may be due to light activation of different redox forms of a cryptochrome photopigment. Finally, we relate these findings to earlier studies showing that the pineal organ of newts is the site of the light-dependent magnetic compass and recent neurophysiological evidence showing magnetic field sensitivity in the frog frontal organ (an outgrowth of the pineal).


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Adler K, Taylor DH (1973) Extraocular perception of polarized light by orienting salamanders. J Comp Physiol 87:203–212
Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W (2007) Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 225:615–624. doi:10.1007/s00425-006-0383-0
Bailey MJ, Chong NW, Xiong J, Cassone VM (2002) Chickens’ Cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors. FEBS Lett 513:169–174
Banerjee R, Schleicher E, Meier S, Viana RM, Pokorny R, Ahmad M, Bittl R, Batschauer A (2007) The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J Biol Chem 282:14916–14922. doi:10.1074/jbc.M700616200
Batschelet E (1981) Circular statistics in biology. Academic, New York
Biskup T, Schleicher E, Okafuji A, Link G, Hitomi K, Getzoff Elizabeth D, Weber S (2009) Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angew Chem Int Ed 48:404–407. doi:10.1002/anie.200803102
Borsuk-Białynicka M, Evans SE (2002) The scapulocoracoid of an Early Triassic stem-frog from Poland. Acta Palaeontol Pol 47:79–96
Bouly JP, Schleicher E, Dionisio-Sese M, Vandenbussche F, Van Der Straeten D, Bakrim N, Meier S, Batschauer A, Galland P, Bittl R, Ahmad M (2007) Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem 282:9383–9391. doi:10.1074/jbc.M609842200
Busza A, Emery-Le M, Rosbash M, Emery P (2004) Roles of the two Drosophila cryptochrome structural domains in circadian photoreception. Science 304:1503–1506
Cannatella DC, Vieites DR, Zhang P, Wake MH, Wake DB (2009) Amphibians (Lissamphibia). In: Hedges SB, Kumar S (eds) The timetree of life. Oxford University Press, New York, pp 353–356
Cashmore A, Jarillo JA, Wu Y, Liu D (1999) Cryptochromes: blue light receptors for plants and animals. Science 284:760–765
Cintolesi F, Ritz T, Kay CWM, Timmel CR, Hore PJ (2003) Anisotropic recombination of an immobilized photoinduced radical pair in a 50-μT magnetic field: a model avian photomagnetoreceptor. Chem Phys 294:385–399. doi:10.1016/S0301-0104(03)00320-3
Deutschlander ME, Borland SC, Phillips JB (1999a) Extraocular magnetic compass in newts. Nature 400:324–325. doi:10.1038/22450
Deutschlander ME, Phillips JB, Borland SC (1999b) The case for light-dependent magnetic orientation in animals. J Exp Biol 202:891–908
Deutschlander ME, Phillips JB, Borland SC (2000) Magnetic compass orientation in the eastern red-spotted newt, Notophthalmus viridescens: rapid acquisition of the shoreward axis. Copeia 2000:413–419
Diego-Rasilla FJ (2003) Homing ability and sensitivity to the geomagnetic field in the alpine newt, Triturus alpestris. Ethol Ecol Evol 15:251–259
Diego-Rasilla FJ, Phillips JB (2007) Magnetic compass orientation in larval Iberian green frogs, Pelophylax perezi. Ethology 113:1–6. doi:10.1111/j.1439-0310.2007.01334.x
Dodt E, Heerd E (1962) Mode of action of pineal nerve fibers in frogs. J Neurophysiol 25:405–429
Eldred WD, Nolte J (1978) Pineal photoreceptors: evidence for a vertebrate visual pigment with two physiologically active states. Vis Res 18:29–32. doi:10.1016/0042-6989(78)90073-1
Ferguson DE, Landreth HF (1966) Celestial orientation of Fowler’s toad (Bufo fowleri). Behaviour 26:105–123
Ferguson DE, Landreth HF, McKeown JP (1967) Sun compass orientation of the northern cricket frog, Acris crepitans. Anim Behav 15:45–53
Freake MJ, Phillips JB (2005) Light-dependent shift in bullfrog tadpole magnetic compass orientation: evidence for a common magnetoreception mechanism in anuran and urodele amphibians. Ethology 111:241–254. doi:10.1111/j.1439-0310.2004.01067.x
Freake MJ, Borland SC, Phillips JB (2002) Use of a magnetic compass for Y-axis orientation in larval bullfrogs (Rana catesbeiana). Copeia 2002:466–471
Gegear RJ, Casselman A, Waddell S, Reppert SM (2008) Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 454:1014–1018. doi:10.1038/nature07183
Giovani B, Byrdin M, Ahmad M, Brettel K (2003) Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat Struct Biol 10:489–490
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gradstein FM, Ogg JG, Smith AG, Agterberg FP, Bleeker W, Cooper RA, Davydov V, Gibbard P, Hinnov LA, House MR, Lourens L, Luterbacher HP, McArthur J, Melchin MJ, Robb LJ, Shergold J, Villeneuve M, Wardlaw BR, Ali J, Brinkhuis H, Hilgen FJ, Hooker J, Howarth RJ, Knoll AH, Laskar J, Monechi S, Plumb KA, Powell J, Raffi I, Röhl U, Sadler P, Sanfilippo A, Schmitz B, Shackleton NJ, Shields GA, Strauss H, Dam JV, Tv K, Veizer J, Wilson DM (2004) A geologic time scale. Cambridge University Press, Cambridge
Hoang N, Schleicher E, Kacprzak S, Bouly J-P, Picot M, Wu W, Berndt A, Wolf E, Bittl R, Ahmad M (2008) Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol 6:e160. doi:10.1371/journal.pbio.0060160
Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F (2004) Novel features of cryptochrome-mediated photoreception in the brain of the circadian clock of Drosophila. J Neurosci 24:1468–1477
Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, Tamotsu S, Terakita A (2004) Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci USA 101:6687–6691. doi:10.1073/pnas.0400819101
Kyriacou CP (2009) Clocks, cryptochromes and Monarch migrations. J Biol 8:55.3–55.4. doi:10.1186/jbiol153
Landreth HF, Ferguson DE (1967) Newts: sun-compass orientation. Science 158:1459–1461
Landreth HF, Ferguson DE (1968) The sun compass of Fowler’s toad, Bufo woodhousei fowleri. Behaviour 30:27–43
Lee MSY, Anderson JS (2006) Molecular clocks and the origin(s) of modern amphibians. Mol Phylogenet Evol 40:635–639
Liedvogel M, Mouritsen H (2010) Cryptochromes—a potential magnetoreceptor: what do we know and what do we want to know? J R Soc Interface 7:S147–S162. doi:10.1098/rsif.2009.0411.focus
Liedvogel M, Maeda K, Henbest K, Schleicher E, Simon T, Timmel CR, Hore PJ, Mouritsen H (2007) Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE 2:e1106. doi:10.1371/journal.pone.0001106
Lin C (2004) Photoreceptors and associated signaling II: cryptochromes. In: Encyclopedia of plant and crop science. University of California, Los Angeles, California, USA
Mardia KV, Jupp PE (2000) Directional statistics. Wiley, New York
Marjanović D, Laurin M (2007) Fossils, molecules, divergence times, and the origin of Lissamphibians. Syst Biol 56:369–388
Milner AR (1990) The radiations of temnospondyl amphibians. In: Taylor PD, Larwood GP (eds) Major evolutionary radiations. Clarendon, Oxford, pp 321–349
Möller A, Sagasser S, Wiltschko W, Schierwater B (2004) Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91:585–588. doi:10.1007/s00114-004-0578-9
Mouritsen H, Ritz T (2005) Magnetoreception and its use in bird navigation. Curr Opin Neurobiol 15:406–414
Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, Dirks P, Weiler R (2004) Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc Natl Acad Sci USA 101:14294–14299. doi:10.1073/pnas.0405968101
Öztürk N, Song S-H, Selby CP, Sancar A (2008) Animal type 1 cryptochromes: analysis of the redox state of the flavin cofactor by site-directed mutagenesis. J Biol Chem 283:3256–3263. doi:10.1074/jbc.M708612200
Phillips JB (1986) Magnetic compass orientation in the Eastern red-spotted newt (Notophthalmus viridescens). J Comp Physiol A 158:103–109
Phillips JB, Borland SC (1992a) Behavioral evidence for the use of a light-dependent magnetoreception mechanism by a vertebrate. Nature 359:142–144. doi:10.1038/359142a0
Phillips JB, Borland SC (1992b) Magnetic compass orientation is eliminated under near-infrared light in the Eastern red-spotted newt (Notophthalmus viridescens). Anim Behav 44:796–797. doi:10.1016/S0003-3472(05)80311-2
Phillips JB, Borland SC (1992c) Wavelength-specific effects of light on magnetic compass orientation of the Eastern red-spotted newt (Notophthalmus viridescens). Ethol Ecol Evol 4:33–42
Phillips JB, Sayeed O (1993) Wavelength-dependent effects of light on magnetic compass orientation in Drosophila melanogaster. J Comp Physiol A 172:303–308. doi:10.1007/BF00216612
Phillips JB, Deutschlander ME, Freake MJ, Borland SC (2001) The role of extraocular photoreceptors in newt magnetic compass orientation: evidence for parallels between light-dependent magnetoreception and polarized light detection in vertebrates. J Exp Biol 204:2543–2552
Phillips JB, Jorge PE, Muheim R (2010) Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanisms. J R Soc Interface 7:S241–S256. doi:10.1098/rsif.2009.0459.focus
Rage JC, Roček Z (1989) Redescription of Triadobatrachus massinoti (Piveteau, 1936) an anuran amphibian from the Early Triassic. Palaeontogr A 206:1–16
Ritz T, Adem S, Schulten K (2000) A model for photoreceptor-based magnetoreception in birds. Biophys J 78:707–718. doi:10.1016/S0006-3495(00)76629-X
Ritz T, Phillips JB, Dommer DH (2002) Shedding light on vertebrate magnetoreception. Neuron 34:503–506. doi:10.1016/S0896-6273(02)00707-9
Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W (2004) Resonance effects indicate a radical pair mechanism for avian magnetic compass. Nature 429:177–180. doi:10.1038/nature02534
Robinson R (2008) Monarchs, Cry2 is king of the clock. PLoS Biol 6:e12
Rodgers CT, Hore PJ (2009) Chemical magnetoreception in birds: the radical pair mechanism. Proc Nat Acad Sci USA 106:353–360. doi:10.1073/pnas.0711968106
Rodríguez-García L, Diego-Rasilla FJ (2006) Use of a magnetic compass for Y-axis orientation in premetamorphic newts (Triturus boscai). J Ethol 24:111–116. doi:10.1007/s10164-005-0169-z
Rosato E, Codd V, Mazzotta G, Piccin A, Zordan M, Costa R, Kyriacou CP (2001) Light-dependent interaction between Drosophila CRY and the clock protein PER mediated by the carboxy terminus of CRY. Curr Biol 11:909–917
Rubens SM (1945) Cube-surface coil for producing a uniform magnetic field. Rev Sci Instrum 16:243–245
Russell AP, Bauer AM, Johnson MK (2005) Migration in amphibians and reptiles: an overview of patterns and orientation mechanisms in relation to life history strategies. In: Elewa AMT (ed) Migration of organisms. Climate. Geography. Ecology. Springer, Berlin, pp 151–203
Ruta M, Coates MI, Quicke DDL (2003) Early tetrapod relationships revisited. Biol Rev 78:251–345
Sancar A (2004) Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem 279:34079–34082. doi:10.1074/jbc.R400016200
Schoch RR, Milner AR (2004) Structure and implications of theories on the origin of lissamphibians. In: Arratia G, Wilson MVH, Cloutier R (eds) Recent advances in the origin and early radiations of vertebrates. Dr. Friedrich Pfeil, Munich, pp 345–377
Schulten K (1982) Magnetic field effects in chemistry and biology. In: Treusch J (ed) Festkörperprobleme [Advances in solid state physics], vol 22. Vieweg, Braunschweig, pp 61–83
Schulten K, Windemuth A (1986) Model for a physiological magnetic compass. In: Maret G, Boccara N, Kiepenheuer J (eds) Biophysical effects of steady magnetic fields. Springer, Berlin, pp 99–106
Schulten K, Swenberg CE, Weller A (1978) A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z Phys Chem NF 111:1–5
Solov’yov IA, Schulten K (2009) Magnetoreception through cryptochrome may involve superoxide. Biophys J 96:4804–4813. doi:10.1016/j.bpj.2009.03.048
Solov’yov IA, Chandler D, Schulten K (2007) Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys J 92:2711–2726
Song S-H, Öztürk N, Denaro TR, NÃz A, Kao Y-T, Zhu H, Zhong D, Reppert SM, Sancar A (2007) Formation and function of flavin anion radical in cryptochrome 1 blue-light photoreceptor of monarch butterfly. J Biol Chem 282:17608–17612. doi:10.1074/jbc.M702874200
Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC (1998) The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95:681–692. doi:10.1016/S0092-8674(00)81638-4
Stebbins RC, Cohen NW (1997) A natural history of amphibians. Princeton University Press, Princeton
Taylor DH (1972) Extra-optic photoreception and compass orientation in larval and adult salamanders (Ambystoma tigrinum). Anim Behav 20:233–236
Taylor DH, Adler K (1973) Spatial orientation by salamanders using plane-polarized light. Science 181:285–287
Taylor DH, Auburn J (1978) Orientation of amphibians by linearly polarized light. In: Schmidt-Koenig K, Keeton W (eds) Animal migration, navigation and homing. Springer, Berlin, pp 334–346
Taylor DH, Ferguson DE (1970) Extraoptic celestial orientation in the southern cricket frog Acris gryllus. Science 168:390–392
Trueb L, Cloutier R (1991) A phylogenetic investigation of the inter- and intrarelationships of the Lissamphibia (Amphibia: Temnospondyli). In: Schultze H-P, Trueb L (eds) Origins of the higher groups of Tetrapods—controversy and consensus. Cornell University Press, Ithaca, pp 223–313
Tu DC, Batten ML, Palczewski K, Van Gelder RN (2004) Nonvisual photoreception in the chick iris. Science 306:129–131. doi:10.1126/science.1101484
van der Horst GTJ, Muijtjens M, Kobayashi K, Takano R, Kanno S-I, Takao M, Jd W, Verkerk A, Eker APM, Dv L, Buijs R, Bootsma D, Hoeijmakers JHJ, Yasui A (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398:627–630. doi:10.1038/19323
van Gelder RN, Wee R, Lee JA, Tu DC (2003) Reduced pupillary light responses in mice lacking cryptochromes. Science 299:222
Van Vickle-Chavez SJ, Van Gelder RN (2007) Action spectrum of Drosophila cryptochrome. J Biol Chem 282:10561–10566. doi:10.1074/jbc.M609314200
Wells DK (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wiltschko R, Wiltschko W (2006) Magnetoreception. BioEssays 28:157–168
Yoshii T, Ahmad M, Helfrich-Förster C (2009) Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol 7:e1000086. doi:10.1371/journal.pbio.1000086
Zhang P, Wake DB (2009) Higher-level salamander relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylogenet Evol 53:492–508
Zhu H, Green CB (2001) Three cryptochromes are rhythmically expressed in Xenopus laevis retinal photoreceptors. Mol Vis 7:210
Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert SM (2008) Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol 6:e4. doi:10.1371/journal.pbio.0060004
Zikihara K, Ishikawa T, Todo T, Tokutomi S (2008) Involvement of electron transfer in the photoreaction of zebrafish cryptochrome-DASH. Photochem Photobiol 84:1016–1023. doi:10.1111/j.1751-1097.2007.00364.x
Acknowledgments
We thank Marcos Diego-Gutiérrez for his valuable assistance during the study. Review by Michael Painter, Rachel Muheim and Paulo Jorge improved the manuscript. We thank three anonymous reviewers for their comments on the manuscript. The Cantabria autonomous government granted the necessary permits for the study. J.B.P. was supported by NSF IOB06-47188 during the preparation of this manuscript. The experiments reported herein comply with the current laws of Spain. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diego-Rasilla, F.J., Luengo, R.M. & Phillips, J.B. Light-dependent magnetic compass in Iberian green frog tadpoles. Naturwissenschaften 97, 1077–1088 (2010). https://doi.org/10.1007/s00114-010-0730-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0730-7