Abstract
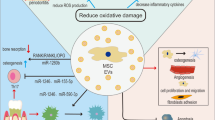
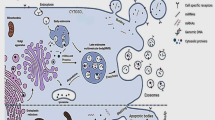
Periodontitis is a chronic inflammatory disease that destroys tooth-supporting structures and poses significant public health challenges due to its high prevalence and links to systemic health conditions. Traditional treatments are effective in reducing the inflammatory response and improving the clinical symptoms of periodontitis. However, these methods are challenging to achieve an ideal treatment effect in alveolar bone repair. Mesenchymal stem cells (MSCs) represent a potential alternative for the treatment of periodontal bone defects due to their self-renewal and differentiation capabilities. Recent research indicates that MSCs exert their effects primarily through paracrine mechanisms. Mesenchymal stem cell–derived extracellular vesicles (MSC-EVs) serve as pivotal mediators in intercellular communication, transferring microRNAs (miRNAs), messenger RNAs (mRNAs), proteins, and cytokines to recipient cells, thereby emulating the therapeutic effects of MSCs. In periodontitis, MSC-EVs play a pivotal role in immunomodulation and bone remodeling, thereby facilitating periodontal bone repair. As a cell-free therapy, MSC-EVs demonstrate considerable clinical potential due to their specialized membrane structure, minimal immunogenicity, low toxicity, high biocompatibility, and nanoscale size. This review indicates that MSC-EVs represent a promising approach for periodontitis treatment, with the potential to overcome the limitations of traditional therapies and offer a more effective solution for bone repair in periodontal disease. In this review, we introduce MSC-EVs, emphasizing their mechanisms and clinical applications in periodontal bone repair. It synthesizes recent advances, existing challenges, and future prospects to present up-to-date information and novel techniques for periodontal regeneration and to guide the improvement of MSC-EV therapy in clinical practice.




Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Hajishengallis G, Korostoff JM (2017) Revisiting the Page & Schroeder model: the good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol 2000 75:116–151. https://doi.org/10.1111/prd.12181
Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15:30–44. https://doi.org/10.1038/nri3785
Pan W, Wang Q, Chen Q (2019) The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci 11:30. https://doi.org/10.1038/s41368-019-0064-z
Hajishengallis G, Chavakis T (2021) Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 21:426–440. https://doi.org/10.1038/s41577-020-00488-6
Slots J (2017) Periodontitis: facts, fallacies and the future. Periodontol 2000 75:7–23. https://doi.org/10.1111/prd.12221
Graziani F, Karapetsa D, Alonso B, Herrera D (2017) Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol 2000 75:152–188. https://doi.org/10.1111/prd.12201
Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS (2020) Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol 47(Suppl 22):4–60. https://doi.org/10.1111/jcpe.13290
Isola G, Polizzi A, Santonocito S, Alibrandi A, Pesce P, Kocher T (2024) Effect of quadrantwise versus full-mouth subgingival instrumentation on clinical and microbiological parameters in periodontitis patients: a randomized clinical trial. J Periodontal Res 59:647–656. https://doi.org/10.1111/jre.13279
Isola G, Pesce P, Polizzi A, Lo Giudice A, Cicciù M, Scannapieco FA (2024) Effects of minimally invasive non-surgical therapy on C-reactive protein, lipoprotein-associated phospholipase A(2), and clinical outcomes in periodontitis patients: a 1-year randomized, controlled clinical trial. J Periodontol 95:949–962. https://doi.org/10.1002/jper.23-0518
Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S (2018) Tissue engineered constructs for periodontal regeneration: current status and future perspectives. Adv Healthc Mater 7:e1800457. https://doi.org/10.1002/adhm.201800457
Amato M, Santonocito S, Viglianisi G, Tatullo M, Isola G (2022) Impact of oral mesenchymal stem cells applications as a promising therapeutic target in the therapy of periodontal disease. Int J Mol Sci 23. https://doi.org/10.3390/ijms232113419
Hernández-Monjaraz B, Santiago-Osorio E, Monroy-García A, Ledesma-Martínez E, Mendoza-Núñez VM (2018) Mesenchymal stem cells of dental origin for inducing tissue regeneration in periodontitis: a mini-review. Int J Mol Sci 19. https://doi.org/10.3390/ijms19040944
Zhuang WZ, Lin YH, Su LJ, Wu MS, Jeng HY, Chang HC, Huang YH, Ling TY (2021) Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci 28:28. https://doi.org/10.1186/s12929-021-00725-7
Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V (2019) Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 8. https://doi.org/10.3390/cells8121605
Tsiapalis D, O'Driscoll L (2020) Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells 9. https://doi.org/10.3390/cells9040991
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. https://doi.org/10.1038/nrm.2017.125
Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R (2016) The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy 18:13–24. https://doi.org/10.1016/j.jcyt.2015.10.008
Hua S, Bartold PM, Gulati K, Moran CS, Ivanovski S, Han P (2021) Periodontal and dental pulp cell-derived small extracellular vesicles: a review of the current status. Nanomaterials (Basel) 11. https://doi.org/10.3390/nano11071858
Liu C, Li Y, Han G (2022) Advances of mesenchymal stem cells released extracellular vesicles in periodontal bone remodeling. DNA Cell Biol 41:935–950. https://doi.org/10.1089/dna.2022.0359
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J et al (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066. https://doi.org/10.3402/jev.v4.27066
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. https://doi.org/10.1080/20013078.2018.1535750
Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902
Lu Y, Mai Z, Cui L, Zhao X (2023) Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res Ther 14:55. https://doi.org/10.1186/s13287-023-03275-x
Krylova SV, Feng D (2023) The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci 24. https://doi.org/10.3390/ijms24021337
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367. https://doi.org/10.1126/science.aau6977
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8:237–255. https://doi.org/10.7150/thno.21945
Isaac R, Reis FCG, Ying W, Olefsky JM (2021) Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab 33:1744–1762. https://doi.org/10.1016/j.cmet.2021.08.006
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. https://doi.org/10.1186/s13578-019-0282-2
Hade MD, Suire CN, Suo Z (2021) Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10. https://doi.org/10.3390/cells10081959
Li XX, Yang LX, Wang C, Li H, Shi DS, Wang J (2023) The roles of exosomal proteins: classification, function, and applications. Int J Mol Sci 24. https://doi.org/10.3390/ijms24043061
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13:17–24. https://doi.org/10.1016/j.gpb.2015.02.001
Skotland T, Sandvig K, Llorente A (2017) Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66:30–41. https://doi.org/10.1016/j.plipres.2017.03.001
Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, Lim LP, Lim SK, Toh WS (2019) Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater 89:252–264. https://doi.org/10.1016/j.actbio.2019.03.021
Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ (2006) Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20:1487–1495. https://doi.org/10.1038/sj.leu.2404296
Georgatzakou HT, Fortis SP, Papageorgiou EG, Antonelou MH, Kriebardis AG (2022) Blood cell-derived microvesicles in hematological diseases and beyond. Biomolecules 12. https://doi.org/10.3390/biom12060803
Wilhelm EN, Mourot L, Rakobowchuk M (2018) Exercise-derived microvesicles: a review of the literature. Sports Med 48:2025–2039. https://doi.org/10.1007/s40279-018-0943-z
Laberge A, Arif S, Moulin VJ (2018) Microvesicles: intercellular messengers in cutaneous wound healing. J Cell Physiol 233:5550–5563. https://doi.org/10.1002/jcp.26426
Zou X, Lei Q, Luo X, Yin J, Chen S, Hao C, Shiyu L, Ma D (2023) Advances in biological functions and applications of apoptotic vesicles. Cell Commun Signal 21:260. https://doi.org/10.1186/s12964-023-01251-9
Zhu Y, Chen X, Liao Y (2023) Mesenchymal stem cells-derived apoptotic extracellular vesicles (ApoEVs): mechanism and application in tissue regeneration. Stem Cells 41:837–849. https://doi.org/10.1093/stmcls/sxad046
Xu X, Lai Y, Hua ZC (2019) Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep 39. https://doi.org/10.1042/bsr20180992
Zhou M, Li YJ, Tang YC, Hao XY, Xu WJ, Xiang DX, Wu JY (2022) Apoptotic bodies for advanced drug delivery and therapy. J Control Release 351:394–406. https://doi.org/10.1016/j.jconrel.2022.09.045
Yu L, Zhu G, Zhang Z, Yu Y, Zeng L, Xu Z, Weng J, Xia J, Li J, Pathak JL (2023) Apoptotic bodies: bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J Nanobiotechnology 21:218. https://doi.org/10.1186/s12951-023-01969-1
Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES (2017) Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8:14128. https://doi.org/10.1038/ncomms14128
Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IK (2015) A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 6:7439. https://doi.org/10.1038/ncomms8439
Wu Y, Zhang Y, Dai L, Wang Q, Xue L, Su Z, Zhang C (2019) An apoptotic body-biomimic liposome in situ upregulates anti-inflammatory macrophages for stabilization of atherosclerotic plaques. J Control Release 316:236–249. https://doi.org/10.1016/j.jconrel.2019.10.043
Poon IKH, Parkes MAF, Jiang L, Atkin-Smith GK, Tixeira R, Gregory CD, Ozkocak DC, Rutter SF, Caruso S, Santavanond JP et al (2019) Moving beyond size and phosphatidylserine exposure: evidence for a diversity of apoptotic cell-derived extracellular vesicles in vitro. J Extracell Vesicles 8:1608786. https://doi.org/10.1080/20013078.2019.1608786
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345. https://doi.org/10.1038/35070009
Park SJ, Kim JM, Kim J, Hur J, Park S, Kim K, Shin HJ, Chwae YJ (2018) Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proc Natl Acad Sci U S A 115:E11721-e11730. https://doi.org/10.1073/pnas.1811432115
Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Béland C et al (2015) The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med 7:318ra200. https://doi.org/10.1126/scitranslmed.aac9816
Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G (2011) Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc Natl Acad Sci U S A 108:20684–20689. https://doi.org/10.1073/pnas.1116848108
Yu L, Dou G, Kuang H, Bao L, Liu H, Ye Q, Wang Z, Yang X, Ren L, Li Z et al (2024) Apoptotic extracellular vesicles induced endothelial cell-mediated autologous stem cell recruitment dominates allogeneic stem cell therapeutic mechanism for bone repair. ACS Nano 18:8718–8732. https://doi.org/10.1021/acsnano.3c11050
Greening DW, Xu R, Ale A, Hagemeyer CE, Chen W (2023) Extracellular vesicles as next generation immunotherapeutics. Semin Cancer Biol 90:73–100. https://doi.org/10.1016/j.semcancer.2023.02.002
Wang Y, Liu S, Li L, Li L, Zhou X, Wan M, Lou P, Zhao M, Lv K, Yuan Y et al (2022) Peritoneal M2 macrophage-derived extracellular vesicles as natural multitarget nanotherapeutics to attenuate cytokine storms after severe infections. J Control Release 349:118–132. https://doi.org/10.1016/j.jconrel.2022.06.063
Guo L, Lai P, Wang Y, Huang T, Chen X, Luo C, Geng S, Huang X, Wu S, Ling W et al (2019) Extracellular vesicles from mesenchymal stem cells prevent contact hypersensitivity through the suppression of Tc1 and Th1 cells and expansion of regulatory T cells. Int Immunopharmacol 74:105663. https://doi.org/10.1016/j.intimp.2019.05.048
Sima C, Viniegra A, Glogauer M (2019) Macrophage immunomodulation in chronic osteolytic diseases-the case of periodontitis. J Leukoc Biol 105:473–487. https://doi.org/10.1002/jlb.1ru0818-310r
Funes SC, Rios M, Escobar-Vera J, Kalergis AM (2018) Implications of macrophage polarization in autoimmunity. Immunology 154:186–195. https://doi.org/10.1111/imm.12910
Schlundt C, Fischer H, Bucher CH, Rendenbach C, Duda GN, Schmidt-Bleek K (2021) The multifaceted roles of macrophages in bone regeneration: a story of polarization, activation and time. Acta Biomater 133:46–57. https://doi.org/10.1016/j.actbio.2021.04.052
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604. https://doi.org/10.1016/j.immuni.2010.05.007
Zhuang Z, Yoshizawa-Smith S, Glowacki A, Maltos K, Pacheco C, Shehabeldin M, Mulkeen M, Myers N, Chong R, Verdelis K et al (2019) Induction of M2 macrophages prevents bone loss in murine periodontitis models. J Dent Res 98:200–208. https://doi.org/10.1177/0022034518805984
Hienz SA, Paliwal S, Ivanovski S (2015) Mechanisms of bone resorption in periodontitis. J Immunol Res 2015:615486. https://doi.org/10.1155/2015/615486
Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Lu L, Yao Z, Goodman SB (2019) Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 196:80–89. https://doi.org/10.1016/j.biomaterials.2017.12.025
Kang M, Huang CC, Lu Y, Shirazi S, Gajendrareddy P, Ravindran S, Cooper LF (2020) Bone regeneration is mediated by macrophage extracellular vesicles. Bone 141:115627. https://doi.org/10.1016/j.bone.2020.115627
Qiao X, Tang J, Dou L, Yang S, Sun Y, Mao H, Yang D (2023) Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomed 18:4683–4703. https://doi.org/10.2147/ijn.S420967
Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, Lin Z (2020) Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater 5:1113–1126. https://doi.org/10.1016/j.bioactmat.2020.07.002
Kang M, Huang CC, Gajendrareddy P, Lu Y, Shirazi S, Ravindran S, Cooper LF (2022) Extracellular vesicles from TNFα preconditioned MSCs: effects on immunomodulation and bone regeneration. Front Immunol 13:878194. https://doi.org/10.3389/fimmu.2022.878194
Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, Chen C, Liu D, Watanabe Y, Hayashi C et al (2021) Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater 122:306–324. https://doi.org/10.1016/j.actbio.2020.12.046
Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, Tian W (2021) Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng Part A 27:962–976. https://doi.org/10.1089/ten.TEA.2020.0141
Ye Q, Xu H, Liu S, Li Z, Zhou J, Ding F, Zhang X, Wang Y, Jin Y, Wang Q (2022) Apoptotic extracellular vesicles alleviate Pg-LPS induced inflammatory responses of macrophages via AMPK/SIRT1/NF-κB pathway and inhibit osteoclast formation. J Periodontol 93:1738–1751. https://doi.org/10.1002/jper.21-0657
Iberg CA, Jones A, Hawiger D (2017) Dendritic cells as inducers of peripheral tolerance. Trends Immunol 38:793–804. https://doi.org/10.1016/j.it.2017.07.007
Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K, Takayanagi H (2018) Host defense against oral microbiota by bone-damaging T cells. Nat Commun 9:701. https://doi.org/10.1038/s41467-018-03147-6
Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D et al. (2018) A dysbiotic microbiome triggers T(H)17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med 10. https://doi.org/10.1126/scitranslmed.aat0797
Song T, Eirin A, Zhu X, Zhao Y, Krier JD, Tang H, Jordan KL, Woollard JR, Taner T, Lerman A et al (2020) Mesenchymal stem cell-derived extracellular vesicles induce regulatory T cells to ameliorate chronic kidney injury. Hypertension 75:1223–1232. https://doi.org/10.1161/hypertensionaha.119.14546
Jung S, Lee S, Kim HJ, Kim S, Moon JH, Chung H, Kang SJ, Park CG (2023) Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification. Exp Mol Med 55:665–679. https://doi.org/10.1038/s12276-023-00949-7
Kang L, Miao Y, Jin Y, Shen S, Lin X (2023) Exosomal miR-205-5p derived from periodontal ligament stem cells attenuates the inflammation of chronic periodontitis via targeting XBP1. Immun Inflamm Dis 11:e743. https://doi.org/10.1002/iid3.743
Xia Y, Cheng T, Zhang C, Zhou M, Hu Z, Kang F, Liao C (2023) Human bone marrow mesenchymal stem cell-derived extracellular vesicles restore Th17/Treg homeostasis in periodontitis via miR-1246. FASEB J 37:e23226. https://doi.org/10.1096/fj.202300674RR
Feng X, McDonald JM (2011) Disorders of bone remodeling. Annu Rev Pathol 6:121–145. https://doi.org/10.1146/annurev-pathol-011110-130203
Zhang Z, Deng M, Hao M, Tang J (2021) Periodontal ligament stem cells in the periodontitis niche: inseparable interactions and mechanisms. J Leukoc Biol 110:565–576. https://doi.org/10.1002/jlb.4mr0421-750r
Tang HN, Xia Y, Yu Y, Wu RX, Gao LN, Chen FM (2016) Stem cells derived from “inflamed” and healthy periodontal ligament tissues and their sheet functionalities: a patient-matched comparison. J Clin Periodontol 43:72–84. https://doi.org/10.1111/jcpe.12501
Lin C, Yang Y, Wang Y, Jing H, Bai X, Hong Z, Zhang C, Gao H, Zhang L (2022) Periodontal ligament fibroblasts-derived exosomes induced by PGE(2) inhibit human periodontal ligament stem cells osteogenic differentiation via activating miR-34c-5p/SATB2/ERK. Exp Cell Res 419:113318. https://doi.org/10.1016/j.yexcr.2022.113318
Saint-Pastou Terrier C, Gasque P (2017) Bone responses in health and infectious diseases: a focus on osteoblasts. J Infect 75:281–292. https://doi.org/10.1016/j.jinf.2017.07.007
Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9:63. https://doi.org/10.1186/s13287-018-0791-7
Zhao B, Chen Q, Zhao L, Mao J, Huang W, Han X, Liu Y (2022) Periodontal ligament stem cell-derived small extracellular vesicles embedded in Matrigel enhance bone repair through the adenosine receptor signaling pathway. Int J Nanomed 17:519–536. https://doi.org/10.2147/ijn.S346755
Lan Q, Cao J, Bi X, Xiao X, Li D, Ai Y (2023) Curcumin-primed periodontal ligament stem cells-derived extracellular vesicles improve osteogenic ability through the Wnt/β-catenin pathway. Front Cell Dev Biol 11:1225449. https://doi.org/10.3389/fcell.2023.1225449
Lei F, Li M, Lin T, Zhou H, Wang F, Su X (2022) Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater 141:333–343. https://doi.org/10.1016/j.actbio.2021.12.035
Li S, Guan X, Yu W, Zhao Z, Sun Y, Bai Y (2023) Effect of human periodontal ligament stem cell-derived exosomes on cementoblast activity. Oral Dis. https://doi.org/10.1111/odi.14671
Lu J, Yu N, Liu Q, Xie Y, Zhen L (2023) Periodontal ligament stem cell exosomes key to regulate periodontal regeneration by miR-31-5p in mice model. Int J Nanomedicine 18:5327–5342. https://doi.org/10.2147/ijn.S409664
Yan C, Li N, Xiao T, Ye X, Fu L, Ye Y, Xu T, Yu J (2022) Extracellular vesicles from the inflammatory microenvironment regulate the osteogenic and odontogenic differentiation of periodontal ligament stem cells by miR-758-5p/LMBR1/BMP2/4 axis. J Transl Med 20:208. https://doi.org/10.1186/s12967-022-03412-9
Shi W, Guo S, Liu L, Liu Q, Huo F, Ding Y, Tian W (2020) Small extracellular vesicles from lipopolysaccharide-preconditioned dental follicle cells promote periodontal regeneration in an inflammatory microenvironment. ACS Biomater Sci Eng 6:5797–5810. https://doi.org/10.1021/acsbiomaterials.0c00882
Ma L, Rao N, Jiang H, Dai Y, Yang S, Yang H, Hu J (2022) Small extracellular vesicles from dental follicle stem cells provide biochemical cues for periodontal tissue regeneration. Stem Cell Res Ther 13:92. https://doi.org/10.1186/s13287-022-02767-6
Li M, Xing X, Huang H, Liang C, Gao X, Tang Q, Xu X, Yang J, Liao L, Tian W (2022) BMSC-derived ApoEVs promote craniofacial bone repair via ROS/JNK signaling. J Dent Res 101:714–723. https://doi.org/10.1177/00220345211068338
Hu Y, Wang Z, Fan C, Gao P, Wang W, Xie Y, Xu Q (2023) Human gingival mesenchymal stem cell-derived exosomes cross-regulate the Wnt/β-catenin and NF-κB signalling pathways in the periodontal inflammation microenvironment. J Clin Periodontol 50:796–806. https://doi.org/10.1111/jcpe.13798
Wang M, Li J, Ye Y, He S, Song J (2020) SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 111:1–11. https://doi.org/10.1016/j.diff.2019.10.003
Wu J, Chen L, Wang R, Song Z, Shen Z, Zhao Y, Huang S, Lin Z (2019) Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng 5:3561–3571. https://doi.org/10.1021/acsbiomaterials.9b00607
Wei J, Song Y, Du Z, Yu F, Zhang Y, Jiang N, Ge X (2020) Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J Mol Histol 51:455–466. https://doi.org/10.1007/s10735-020-09896-3
Huang CC, Kang M, Leung K, Lu Y, Shirazi S, Gajendrareddy P, Ravindran S (2023) Micro RNA based MSC EV engineering: targeting the BMP2 cascade for bone repair. Front Cell Dev Biol 11:1127594. https://doi.org/10.3389/fcell.2023.1127594
Huang CC, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, Gajendrareddy P, Ravindran S (2020) Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater 109:182–194. https://doi.org/10.1016/j.actbio.2020.04.017
Yang S, Zhu B, Tian XY, Yu HY, Qiao B, Zhao LS, Zhang B (2022) Exosomes derived from human umbilical cord mesenchymal stem cells enhance the osteoblastic differentiation of periodontal ligament stem cells under high glucose conditions through the PI3K/AKT signaling pathway. Biomed Environ Sci 35:811–820. https://doi.org/10.3967/bes2022.105
Li X, Jiang Y, Liu X, Fu J, Du J, Luo Z, Xu J, Bhawal UK, Liu Y, Guo L (2023) Mesenchymal stem cell-derived apoptotic bodies alleviate alveolar bone destruction by regulating osteoclast differentiation and function. Int J Oral Sci 15:51. https://doi.org/10.1038/s41368-023-00255-y
Qu F, Zhang YF, Wang YY, Cao XM, Shen YY, Wu ZA, Wu YQ, Xu C (2024) Cyclic stretch-induced exosomes from periodontal ligament cells promote osteoblasts osteogenic differentiation via the miR-181d-5p/TNF signaling pathway. Arch Oral Biol 157:105843. https://doi.org/10.1016/j.archoralbio.2023.105843
Yang Y, Zhang B, Yang Y, Peng B, Ye R (2022) PLGA containing human adipose-derived stem cell-derived extracellular vesicles accelerates the repair of alveolar bone defects via transfer of CGRP. Oxid Med Cell Longev 2022:4815284. https://doi.org/10.1155/2022/4815284
Baron R, Rawadi G, Roman-Roman S (2006) Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol 76:103–127. https://doi.org/10.1016/s0070-2153(06)76004-5
Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT (2000) The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16:279–283. https://doi.org/10.1016/s0168-9525(00)02028-x
Chen XJ, Shen YS, He MC, Yang F, Yang P, Pang FX, He W, Cao YM, Wei QS (2019) Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed Pharmacother 112:108746. https://doi.org/10.1016/j.biopha.2019.108746
Li Y, Wang X, Lu J (2023) Interleukin-35 promote osteogenesis and inhibit adipogenesis: role of Wnt/β-catenin and PPARγ signaling pathways. Inflammation 46:522–533. https://doi.org/10.1007/s10753-022-01749-3
Sangadala S, Kim CH, Fernandes LM, Makkar P, Beck GR, Boden SD, Drissi H, Presciutti SM (2023) Sclerostin small-molecule inhibitors promote osteogenesis by activating canonical Wnt and BMP pathways. Elife 12. https://doi.org/10.7554/eLife.63402
Xu Y, Jiang Y, Jia B, Wang Y, Li T (2021) Icariin stimulates osteogenesis and suppresses adipogenesis of human bone mesenchymal stem cells via miR-23a-mediated activation of the Wnt/β-catenin signaling pathway. Phytomedicine 85:153485. https://doi.org/10.1016/j.phymed.2021.153485
Boland GM, Perkins G, Hall DJ, Tuan RS (2004) Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93:1210–1230. https://doi.org/10.1002/jcb.20284
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu N, Ding B, Liu W, Liu Y, Shi H et al (2011) High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J Bone Miner Res 26:2082–2095. https://doi.org/10.1002/jbmr.440
Wu M, Chen G, Li YP (2016) TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. https://doi.org/10.1038/boneres.2016.9
Pizzicannella J, Gugliandolo A, Orsini T, Fontana A, Ventrella A, Mazzon E, Bramanti P, Diomede F, Trubiani O (2019) Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front Physiol 10:512. https://doi.org/10.3389/fphys.2019.00512
Yi G, Zhang S, Ma Y, Yang X, Huo F, Chen Y, Yang B, Tian W (2022) Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res Ther 13:41. https://doi.org/10.1186/s13287-022-02721-6
Han XD, Chen HM, Li C (2022) Effect of human periodontal ligament stem cell-derived extracellular vesicles on macrophage pyroptosis and periodontal inflammatory injury in periodontitis. Cells Tissues Organs 211:57–72. https://doi.org/10.1159/000519569
Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 receptor in infection and inflammation. Immunity 47:15–31. https://doi.org/10.1016/j.immuni.2017.06.020
Xu XY, Tian BM, Xia Y, Xia YL, Li X, Zhou H, Tan YZ, Chen FM (2020) Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl Med 9:1414–1430. https://doi.org/10.1002/sctm.19-0418
Sun J, Wang Z, Liu P, Hu Y, Li T, Yang J, Gao P, Xu Q (2022) Exosomes derived from human gingival mesenchymal stem cells attenuate the inflammatory response in periodontal ligament stem cells. Front Chem 10:863364. https://doi.org/10.3389/fchem.2022.863364
Lee HL, Yi T, Baek K, Kwon A, Hwang HR, Qadir AS, Park HJ, Woo KM, Ryoo HM, Kim GS et al (2013) Tumor necrosis factor-α enhances the transcription of Smad ubiquitination regulatory factor 1 in an activating protein-1- and Runx2-dependent manner. J Cell Physiol 228:1076–1086. https://doi.org/10.1002/jcp.24256
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21:421–438. https://doi.org/10.1038/s41580-020-0250-z
Wen ZQ, Lin J, Xie WQ, Shan YH, Zhen GH, Li YS (2023) Insights into the underlying pathogenesis and therapeutic potential of endoplasmic reticulum stress in degenerative musculoskeletal diseases. Mil Med Res 10:54. https://doi.org/10.1186/s40779-023-00485-5
Zheng J, Gao Y, Lin H, Yuan C, Keqianzhi (2021) Enhanced autophagy suppresses inflammation-mediated bone loss through ROCK1 signaling in bone marrow mesenchymal stem cells. Cells Dev 167:203687. https://doi.org/10.1016/j.cdev.2021.203687
He X, Zhang J, Guo Y, Yang X, Huang Y, Hao D (2022) Exosomal miR-9-5p derived from BMSCs alleviates apoptosis, inflammation and endoplasmic reticulum stress in spinal cord injury by regulating the HDAC5/FGF2 axis. Mol Immunol 145:97–108. https://doi.org/10.1016/j.molimm.2022.03.007
Wang C, Zhu G, He W, Yin H, Lin F, Gou X, Li X (2019) BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J 33:5440–5456. https://doi.org/10.1096/fj.201801821R
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342. https://doi.org/10.1038/nature01658
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508. https://doi.org/10.1126/science.289.5484.1504
Kim HY, Song MK, Lim Y, Jang JS, An SJ, Kim HH, Choi BK (2022) Effects of extracellular vesicles derived from oral bacteria on osteoclast differentiation and activation. Sci Rep 12:14239. https://doi.org/10.1038/s41598-022-18412-4
Hayashi C, Fukuda T, Kawakami K, Toyoda M, Nakao Y, Watanabe Y, Shinjo T, Sano T, Iwashita M, Yotsumoto K et al (2022) miR-1260b inhibits periodontal bone loss by targeting ATF6β mediated regulation of ER stress. Front Cell Dev Biol 10:1061216. https://doi.org/10.3389/fcell.2022.1061216
Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J (2017) Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol 44:456–462. https://doi.org/10.1111/jcpe.12732
Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropouloss A (2015) Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol 2000 68:182–216. https://doi.org/10.1111/prd.1208
Rezaie J, Feghhi M, Etemadi T (2022) A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal 20:145. https://doi.org/10.1186/s12964-022-00959-4
Lu Y, Zhao L, Mao J, Liu W, Ma W, Zhao B (2023) Rab27a-mediated extracellular vesicle secretion contributes to osteogenesis in periodontal ligament-bone niche communication. Sci Rep 13:8479. https://doi.org/10.1038/s41598-023-35172-x
Zhou H, Qi YX, Zhu CH, Li A, Pei DD (2023) Mesenchymal stem cell-derived extracellular vesicles for treatment of bone loss within periodontitis in pre-clinical animal models: a meta-analysis. BMC Oral Health 23:701. https://doi.org/10.1186/s12903-023-03398-w
Luo H, Chen D, Li R, Li R, Teng Y, Cao Y, Zou X, Wang W, Zhou C (2023) Genetically engineered CXCR4-modified exosomes for delivery of miR-126 mimics to macrophages alleviate periodontitis. J Nanobiotechnol 21:116. https://doi.org/10.1186/s12951-023-01863-w
Lai S, Deng L, Liu C, Li X, Fan L, Zhu Y, Yang Y, Mu Y (2023) Bone marrow mesenchymal stem cell-derived exosomes loaded with miR-26a through the novel immunomodulatory peptide DP7-C can promote osteogenesis. Biotechnol Lett 45:905–919. https://doi.org/10.1007/s10529-023-03376-w
Huang X, Deng Y, Xiao J, Wang H, Yang Q, Cao Z (2024) Genetically engineered M2-like macrophage-derived exosomes for P. gingivalis-suppressed cementum regeneration: from mechanism to therapy. Bioact Mater 32:473–487. https://doi.org/10.1016/j.bioactmat.2023.10.009
Zhang T, Chen Z, Zhu M, Jing X, Xu X, Yuan X, Zhou M, Zhang Y, Lu M, Chen D et al (2023) Extracellular vesicles derived from human dental mesenchymal stem cells stimulated with low-intensity pulsed ultrasound alleviate inflammation-induced bone loss in a mouse model of periodontitis. Genes Dis 10:1613–1625. https://doi.org/10.1016/j.gendis.2022.06.009
Yin T, Liu Y, Ji W, Zhuang J, Chen X, Gong B, Chu J, Liang W, Gao J, Yin Y (2023) Engineered mesenchymal stem cell-derived extracellular vesicles: a state-of-the-art multifunctional weapon against Alzheimer’s disease. Theranostics 13:1264–1285. https://doi.org/10.7150/thno.81860
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65:336–341. https://doi.org/10.1016/j.addr.2012.07.001
Kim H, Kim D, Nam H, Moon S, Kwon YJ, Lee JB (2020) Engineered extracellular vesicles and their mimetics for clinical translation. Methods 177:80–94. https://doi.org/10.1016/j.ymeth.2019.10.005
Lotfy A, AboQuella NM, Wang H (2023) Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther 14:66. https://doi.org/10.1186/s13287-023-03287-7
Do J, Dc T, Dordevic M (2020) IRB approved pilot safety study of an extracellular vesicle isolate product evaluating the treatment of osteoarthritis in combat-related injuries. J Stem Cell Res 1:1–10. https://doi.org/10.52793/JSCR.2020.1(2)-09
Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, Peng Q (2019) Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 86:1–14. https://doi.org/10.1016/j.actbio.2018.12.045
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614. https://doi.org/10.1038/mt.2010.105
Shekari F, Abyadeh M, Meyfour A, Mirzaei M, Chitranshi N, Gupta V, Graham SL, Salekdeh GH (2023) Extracellular vesicles as reconfigurable therapeutics for eye diseases: promises and hurdles. Prog Neurobiol 225:102437. https://doi.org/10.1016/j.pneurobio.2023.102437
Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G (2014) Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal 12:26. https://doi.org/10.1186/1478-811x-12-26
Zheng W, He R, Liang X, Roudi S, Bost J, Coly PM, van Niel G, Andaloussi SEL (2022) Cell-specific targeting of extracellular vesicles through engineering the glycocalyx. J Extracell Vesicles 11:e12290. https://doi.org/10.1002/jev2.12290
Gupta D, Zickler AM, El Andaloussi S (2021) Dosing extracellular vesicles. Adv Drug Deliv Rev 178:113961. https://doi.org/10.1016/j.addr.2021.113961
Visan KS, Wu LY, Voss S, Wuethrich A, Möller A (2023) Status quo of extracellular vesicle isolation and detection methods for clinical utility. Semin Cancer Biol 88:157–171. https://doi.org/10.1016/j.semcancer.2022.12.008
Levy D, Jeyaram A, Born LJ, Chang KH, Abadchi SN, Hsu ATW, Solomon T, Aranda A, Stewart S, He X et al (2023) Impact of storage conditions and duration on function of native and cargo-loaded mesenchymal stromal cell extracellular vesicles. Cytotherapy 25:502–509. https://doi.org/10.1016/j.jcyt.2022.11.006
Wu JY, Li YJ, Hu XB, Huang S, Xiang DX (2021) Preservation of small extracellular vesicles for functional analysis and therapeutic applications: a comparative evaluation of storage conditions. Drug Deliv 28:162–170. https://doi.org/10.1080/10717544.2020.1869866
Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R (2018) To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol 9:1199. https://doi.org/10.3389/fphar.2018.01199
Thone MN, Kwon YJ (2020) Extracellular blebs: artificially-induced extracellular vesicles for facile production and clinical translation. Methods 177:135–145. https://doi.org/10.1016/j.ymeth.2019.11.007
Funding
This work was supported by grants from the National Natural Science Foundation of China (32271365) and the Key Project of Sichuan Province (Grant No. 2023YFS0151).
Author information
Authors and Affiliations
Contributions
All authors contributed to the article conception and design. Methodology, investigation, and visualization were performed by Mengbing Chen, Bo Huang, and Xiaoxia Su. The first draft of the manuscript was written by Mengbing Chen and Bo Huang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, M., Huang, B. & Su, X. Mesenchymal stem cell–derived extracellular vesicles in periodontal bone repair. J Mol Med 103, 137–156 (2025). https://doi.org/10.1007/s00109-025-02513-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-025-02513-4