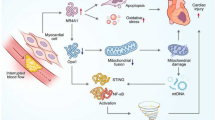
Abstract
Mitochondria consist of the inner mitochondrial membrane and the outer mitochondrial membrane, which maintain mitochondrial homeostasis through continuous fission and fusion to ensure a healthy mitochondrial network and thus regulate normal cellular function, namely mitochondrial dynamics. The imbalance between mitochondrial fusion and fission results in abnormal mitochondrial structure and eventually mitochondrial dysfunction, which is involved in the pathological process of ischemia-reperfusion injury (IRI). Optic atrophy 1 (OPA1) is a key protein that regulates mitochondrial inner membrane fusion and ensures normal mitochondrial function by balancing mitochondrial dynamics, participating in various processes such as mitochondrial fusion, oxidative stress, and apoptosis. Ischemia-induced changes in mitochondrial dynamics may be a key factor in limiting the recanalization time window and exacerbating reperfusion injury, and the mechanisms of these changes deserve further attention. Therefore, targeting OPA1-related mitochondrial fusions, thereby balancing mitochondrial dynamics and improving mitochondrial dysfunction, is a promising therapeutic strategy for ischemia-reperfusion diseases. This review will elaborate on the structure and function of OPA1 and the role of OPA1 in IRI to provide promising therapeutic targets for the treatment of ischemia-reperfusion diseases.

Similar content being viewed by others
Availability of data and material
Not applicable.
References
Cho HM, Sun W (2020) Molecular cross talk among the components of the regulatory machinery of mitochondrial structure and quality control. Exp Mol Med 52(5):730–737
Chan DC (2020) Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol 15:235–259
Benador IY, Veliova M, Liesa M et al (2019) Mitochondria bound to lipid droplets: where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab 29(4):827–835
Catanese L, Tarsia J, Fisher M (2017) Acute ischemic stroke therapy overview. Circ Res 120(3):541–558
Pundik S, Xu K, Sundararajan S (2012) Reperfusion brain injury: focus on cellular bioenergetics. Neurology 79(13 Suppl 1):S44-51
Kulek AR, Anzell A, Wider JM et al (2020) Mitochondrial quality control: role in cardiac models of lethal ischemia-reperfusion injury. Cells 9(1):214
Macvicar T, Langer T (2016) OPA1 processing in cell death and disease - the long and short of it. J Cell Sci 129(12):2297–2306
Li L, Martin-Levilain J, Jimenez-Sanchez C et al (2019) In vivo stabilization of OPA1 in hepatocytes potentiates mitochondrial respiration and gluconeogenesis in a prohibitin-dependent way. J Biol Chem 294(34):12581–12598
Giacomello M, Pyakurel A, Glytsou C et al (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21(4):204–224
Tilokani L, Nagashima S, Paupe V et al (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62(3):341–360
Di Nottia M, Verrigni D, Torraco A et al (2021) Mitochondrial dynamics: molecular mechanisms, related primary mitochondrial disorders and therapeutic approaches. Genes 12(2):247
Sun Y, Xue W, Song Z et al (2016) Restoration of Opa1-long isoform inhibits retinal injury-induced neurodegeneration. J Mol Med 94(3):335–346
Gilkerson R, De La Torre P, St VS (2021) Mitochondrial OMA1 and OPA1 as gatekeepers of organellar structure/function and cellular stress response. Front Cell Dev Biol 9:626117
Yapa NMB, Lisnyak V, Reljic B et al (2021) Mitochondrial dynamics in health and disease. FEBS Lett 595(8):1184–1204
Ban T, Ishihara T, Kohno H et al (2017) Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat Cell Biol 19(7):856–863
Ge Y, Shi X, Boopathy S et al (2020) Two forms of Opa1 cooperate to complete fusion of the mitochondrial inner-membrane. Elife 9:e50973
Delettre C, Lenaers G, Griffoin JM et al (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26(2):207–210
Civiletto G, Varanita T, Cerutti R et al (2015) Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab 21(6):845–854
Noone J, O’gorman DJ, Kenny HC (2022) OPA1 regulation of mitochondrial dynamics in skeletal and cardiac muscle. Trends Endocrinol Metab 33(10):710–721
Gao S, Hu J (2021) Mitochondrial fusion: the machineries in and out. Trends Cell Biol 31(1):62–74
Anzell AR, Maizy R, Przyklenk K et al (2018) Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol 55(3):2547–2564
Cogliati S, Frezza C, Soriano ME et al (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155(1):160–171
Pellegrini L, Scorrano L (2007) A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ 14(7):1275–1284
Chen X, Glytsou C, Zhou H et al (2019) Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov 9(7):890–909
Landes T, Emorine LJ, Courilleau D et al (2010) The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep 11(6):459–465
Wu W, Zhao D, Shah SZA et al (2019) OPA1 overexpression ameliorates mitochondrial cristae remodeling, mitochondrial dysfunction, and neuronal apoptosis in prion diseases. Cell Death Dis 10(10):710
Del Dotto V, Fogazza M, Lenaers G et al (2018) OPA1: how much do we know to approach therapy? Pharmacol Res 131:199–210
De Oliveira LG, Angelo YS, Iglesias AH et al (2021) Unraveling the link between mitochondrial dynamics and neuroinflammation. Front Immunol 12:624919
Yin Y, Shen H (2021) Advances in cardiotoxicity induced by altered mitochondrial dynamics and mitophagy. Front Cardiovasc Med 8:739095
Lei Q, Tan J, Yi S et al (2018) Mitochonic acid 5 activates the MAPK-ERK-yap signaling pathways to protect mouse microglial BV-2 cells against TNFα-induced apoptosis via increased Bnip3-related mitophagy. Cell Mol Biol Lett 23:14
Huang J, Li R, Wang C (2021) The role of mitochondrial quality control in cardiac ischemia/reperfusion injury. Oxid Med Cell Longev 2021:5543452
Cardanho-Ramos C, Morais VA (2021) Mitochondrial biogenesis in neurons: how and where. Int J Mol Sci 22(23):13059
Kalogeris T, Baines CP, Krenz M et al (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317
Wu M, Gu X, Ma Z (2021) Mitochondrial quality control in cerebral ischemia-reperfusion injury. Mol Neurobiol 58(10):5253–5271
Nagaraja N, Forder JR, Warach S et al (2020) Reversible diffusion-weighted imaging lesions in acute ischemic stroke: a systematic review. Neurology 94(13):571–587
Wai T, García-Prieto J, Baker MJ et al (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350(6265):aad0116
Korwitz A, Merkwirth C, Richter-Dennerlein R et al (2016) Loss of OMA1 delays neurodegeneration by preventing stress-induced OPA1 processing in mitochondria. J Cell Biol 212(2):157–166
Xiao X, Hu Y, Quirós PM et al (2014) OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol 306(11):F1318-1326
Ma S, Dong Z (2019) Melatonin attenuates cardiac reperfusion stress by improving OPA1-related mitochondrial fusion in a Yap-Hippo pathway-dependent manner. J Cardiovasc Pharmacol 73(1):27–39
Zhu H, Tan Y, Du W et al (2021) Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol 38:101777
Li X, Li H, Xu Z et al (2022) Ischemia-induced cleavage of OPA1 at S1 site aggravates mitochondrial fragmentation and reperfusion injury in neurons. Cell Death Dis 13(4):321
Wei N, Pu Y, Yang Z et al (2019) Therapeutic effects of melatonin on cerebral ischemia reperfusion injury: role of Yap-OPA1 signaling pathway and mitochondrial fusion. Biomed Pharmacother 110:203–212
Lai Y, Lin P, Chen M et al (2020) Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biol 34:101503
Cai Y, Yang E, Yao X et al (2021) FUNDC1-dependent mitophagy induced by tPA protects neurons against cerebral ischemia-reperfusion injury. Redox Biol 38:101792
Zhang Y, Wang Y, Xu J et al (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res 66(2):e12542
Guan L, Che Z, Meng X et al (2019) MCU up-regulation contributes to myocardial ischemia-reperfusion injury through calpain/OPA-1-mediated mitochondrial fusion/mitophagy inhibition. J Cell Mol Med 23(11):7830–7843
Wang K, Liu Z, Zhao M et al (2020) Kappa-opioid receptor activation promotes mitochondrial fusion and enhances myocardial resistance to ischemia and reperfusion injury via STAT3-OPA1 pathway. Eur J Pharmacol 874:172987
Maneechote C, Palee S, Kerdphoo S et al (2019) Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci 133(3):497–513
Le Page S, Niro M, Fauconnier J et al (2016) Increase in cardiac ischemia-reperfusion injuries in Opa1+/- mouse model. PLoS ONE 11(10):e0164066
Rodríguez-Graciani KM, Chapa-Dubocq XR, Macmillan-Crow LA et al (2020) Association between L-OPA1 cleavage and cardiac dysfunction during ischemia-reperfusion injury in rats. Cell Physiol Biochem 54(6):1101–1114
Chen WR, Zhou YJ, Yang JQ et al (2020) Melatonin attenuates calcium deposition from vascular smooth muscle cells by activating mitochondrial fusion and mitophagy via an AMPK/OPA1 signaling pathway. Oxid Med Cell Longev 2020:5298483
He Z, Ning N, Zhou Q et al (2020) Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med 146:45–58
Ong SB, Kwek XY, Katwadi K et al (2019) Targeting mitochondrial fission using Mdivi-1 in a clinically relevant large animal model of acute myocardial infarction: a pilot study. Int J Mol Sci 20(16):3972
Kalkhoran SB, Kriston-Vizi J, Hernandez-Resendiz S et al (2022) Hydralazine protects the heart against acute ischaemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovasc Res 118(1):282–294
Grohm J, Kim SW, Mamrak U et al (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19(9):1446–1458
Shields LY, Kim H, Zhu L et al (2015) Dynamin-related protein 1 is required for normal mitochondrial bioenergetic and synaptic function in CA1 hippocampal neurons. Cell Death Dis 6(4):e1725
Mishra P, Carelli V, Manfredi G et al (2014) Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 19(4):630–641
Acknowledgements
We thank all individuals who participated in or helped with this research.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81704188) and the Natural Science Research Program of the Hubei Provincial Education Department (No.D20202006).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Y., Zhang, Xm. Mechanistic study of optic atrophy 1 in ischemia-reperfusion disease. J Mol Med 101, 1–8 (2023). https://doi.org/10.1007/s00109-022-02271-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02271-7