Abstract
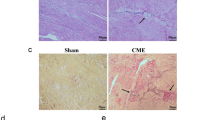
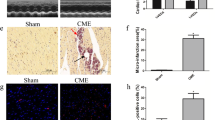
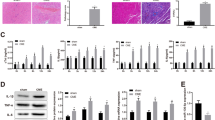
This study aims to explore the mechanism underlying miR-142-3p regulating myocardial injury induced by coronary microembolization (CME) through ATXN1L. miR-142-3p overexpression or ATXN1L knockout adenovirus vectors were injected into rats before CME treatment. Cardiac functions were examined by echocardiography, and pathologies of myocardial tissues were assessed. Then, serum cTnI and IL-1β contents and concentrations of IL-1β and IL-18 in cell supernatant were measured. Immunofluorescence determined the localization of histone deacetylase 3 (HDAC3). The interaction between miR-142-3p and ATXN1L as well as the binding between HDAC3 and histone 3 (H3) was identified. The binding of ATXN1L and HDAC3 to NOL3 promoter was verified using ChIP. The levels of ATXN1L, NOL3, and miR-142-3p as well as apoptosis- and pyroptosis-related proteins and acetyl-histone 3 (ac-H3) were evaluated. CME treatment impaired the cardiac functions in rats and increased cTnI content. CME rats showed microinfarction foci in myocardial tissues. After CME treatment, miR-142-3p and NOL3 were modestly expressed while ATXN1L content was elevated, in addition to increases in apoptosis and pyroptosis. miR-142-3p overexpression or ATXN1L knockout alleviated CME-induced myocardial injury, cardiomyocyte apoptosis, and pyroptosis in myocardial tissues. miR-142-3p regulated ATXN1L expression in a targeted manner. In the cellular context, miR-142-3p overexpression attenuated apoptosis and pyroptosis in cardiomyocytes, which was partly counteracted by ATXN1L overexpression. ATXN1L functioned on cardiomyocytes by promoting deacetylation of H3 through HDAC3 and thus inhibited NOL3 expression. Inhibition of HDAC3 or overexpression of NOL3 ameliorated the promotive effects of ATXN1L on cardiomyocyte apoptosis and pyroptosis. In vivo and in vitro evidence in this study supported that miR-142-3p could attenuate CME-induced myocardial injury via ATXN1L/HDAC3/NOL3.
Highlights
-
CME model witnessed aberrant expression of miR-142-3p, ATXN1L, and NOL3;
-
miR-142-3p negatively regulated ATXN1L;
-
miR-142-3p mediated CME-induced myocardial injury through ATXN1L;
-
ATXN1L promoted deacetylation of H3 through HDAC3 and thus inhibited NOL3 expression;
-
ATXN1L acted on cardiomyocyte apoptosis and pyroptosis through HDAC3/NOL3 axis.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhou Y, Li T, Chen Z, Huang J, Qin Z, Li L (2021) Overexpression of lncRNA TUG1 alleviates NLRP3 inflammasome-mediated cardiomyocyte pyroptosis through targeting the miR-186-5p/XIAP axis in coronary microembolization-induced myocardial damage. Front Immunol 12:637598. https://doi.org/10.3389/fimmu.2021.637598
Wang XT, Wu XD, Lu YX, Sun YH, Zhu HH, Liang JB, He WK, Zeng ZY, Li L (2017) Potential involvement of MiR-30e-3p in myocardial injury induced by coronary microembolization via autophagy activation. Cell Physiol Biochem 44:1995–2004. https://doi.org/10.1159/000485905
Liu T, Zhou Y, Liu YC, Wang JY, Su Q, Tang ZL, Li L (2015) Coronary microembolization induces cardiomyocyte apoptosis through the LOX-1-dependent endoplasmic reticulum stress pathway involving JNK/P38 MAPK. Can J Cardiol 31:1272–1281. https://doi.org/10.1016/j.cjca.2015.01.013
Liu Y, Liu Y, Huang X, Zhang J, Yang L (2019) Protective effects and mechanism of curcumin on myocardial injury induced by coronary microembolization. J Cell Biochem 120:5695–5703. https://doi.org/10.1002/jcb.27854
Wang JY, Chen H, Su X, Zhou Y, Li L (2017) Atorvastatin pretreatment inhibits myocardial inflammation and apoptosis in swine after coronary microembolization. J Cardiovasc Pharmacol Ther 22:189–195. https://doi.org/10.1177/1074248416662348
Zhaolin Z, Guohua L, Shiyuan W, Zuo W (2019) Role of pyroptosis in cardiovascular disease. Cell Prolif 52:e12563. https://doi.org/10.1111/cpr.12563
Chen ZQ, Zhou Y, Chen F, Huang JW, Li HL, Li T, Li L (2021) miR-200a-3p attenuates coronary microembolization-induced myocardial injury in rats by inhibiting TXNIP/NLRP3-mediated cardiomyocyte pyroptosis. Front Cardiovasc Med 8:693257. https://doi.org/10.3389/fcvm.2021.693257
Su Q, Li L, Zhao J, Sun Y, Yang H (2017) MiRNA expression profile of the myocardial tissue of pigs with coronary microembolization. Cell Physiol Biochem 43:1012–1024. https://doi.org/10.1159/000481699
Wang Y, Ouyang M, Wang Q, Jian Z (2016) MicroRNA-142-3p inhibits hypoxia/reoxygenation induced apoptosis and fibrosis of cardiomyocytes by targeting high mobility group box 1. Int J Mol Med 38:1377–1386. https://doi.org/10.3892/ijmm.2016.2756
Su Q, Lv X, Ye Z, Sun Y, Kong B, Qin Z, Li L (2019) The mechanism of miR-142-3p in coronary microembolization-induced myocardiac injury via regulating target gene IRAK-1. Cell Death Dis 10:61. https://doi.org/10.1038/s41419-019-1341-7
Wong D, Lounsbury K, Lum A, Song J, Chan S, LeBlanc V, Chittaranjan S, Marra M, Yip S (2019) Transcriptomic analysis of CIC and ATXN1L reveal a functional relationship exploited by cancer. Oncogene 38:273–290. https://doi.org/10.1038/s41388-018-0427-5
Kahle JJ, Souroullas GP, Yu P, Zohren F, Lee Y, Shaw CA, Zoghbi HY, Goodell MA (2013) Ataxin1L is a regulator of HSC function highlighting the utility of cross-tissue comparisons for gene discovery. PLoS Genet 9:e1003359. https://doi.org/10.1371/journal.pgen.1003359
Wang H, Zhou X, Li H, Qian X, Wang Y, Ma L (2017) Transient receptor potential melastatin 2 negatively regulates LPS-ATP-induced caspase-1-dependent pyroptosis of bone marrow-derived macrophage by modulating ROS production. Biomed Res Int 2017:2975648. https://doi.org/10.1155/2017/2975648
Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G (2015) Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43:923–932. https://doi.org/10.1016/j.immuni.2015.10.009
Zhao LR, Xing RL, Wang PM, Zhang NS, Yin SJ, Li XC, Zhang L (2018) NLRP1 and NLRP3 inflammasomes mediate LPS/ATP induced pyroptosis in knee osteoarthritis. Mol Med Rep 17:5463–5469. https://doi.org/10.3892/mmr.2018.8520
Burja B, Kuret T, Janko T, Topalovic D, Zivkovic L, Mrak-Poljsak K, Spremo-Potparevic B, Zigon P, Distler O, Cucnik S et al (2019) Olive leaf extract attenuates inflammatory activation and DNA damage in human arterial endothelial cells. Front Cardiovasc Med 6:56. https://doi.org/10.3389/fcvm.2019.00056
Venkatraman A, Hu YS, Didonna A, Cvetanovic M, Krbanjevic A, Bilesimo P, Opal P (2014) The histone deacetylase HDAC3 is essential for Purkinje cell function, potentially complicating the use of HDAC inhibitors in SCA1. Hum Mol Genet 23:3733–3745. https://doi.org/10.1093/hmg/ddu081
Karagianni P, Wong J (2007) HDAC3: taking the SMRT-N-CoRrect road to repression. Oncogene 26:5439–5449. https://doi.org/10.1038/sj.onc.1210612
Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C et al (2014) MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5:e1479. https://doi.org/10.1038/cddis.2014.430
Ma J, Qian J, Chang S, Chen Z, Jin H, Zeng M, Zou Y, Ge J (2014) Left ventricular remodeling with preserved function after coronary microembolization: the effect of methylprednisolone. Eur J Med Res 19:7. https://doi.org/10.1186/2047-783X-19-7
Zhang Y, Zhang L, Zheng H, Chen H (2018) Effects of atrial fibrillation on complications and prognosis of patients receiving emergency PCI after acute myocardial infarction. Exp Ther Med 16:3574–3578. https://doi.org/10.3892/etm.2018.6640
Wang W, Ye S, Zhang L, Jiang Q, Chen J, Chen X, Zhang F, Wu H (2020) Granulocyte colony-stimulating factor attenuates myocardial remodeling and ventricular arrhythmia susceptibility via the JAK2-STAT3 pathway in a rabbit model of coronary microembolization. BMC Cardiovasc Disord 20:85. https://doi.org/10.1186/s12872-020-01385-5
Huang C, Qu Y, Feng F, Zhang H, Shu L, Zhu X, Huang G, Xu J (2022) Cardioprotective effect of circ_SMG6 Knockdown against myocardial ischemia/reperfusion injury correlates with miR-138-5p-mediated EGR1/TLR4/TRIF inactivation. Oxid Med Cell Longev 2022:1927260. https://doi.org/10.1155/2022/1927260
Kong B, Qin Z, Ye Z, Yang X, Li L, Su Q (2019) microRNA-26a-5p affects myocardial injury induced by coronary microembolization by modulating HMGA1. J Cell Biochem 120:10756–10766. https://doi.org/10.1002/jcb.28367
Zhu HH, Wang XT, Sun YH, He WK, Liang JB, Mo BH, Li L (2019) MicroRNA-486-5p targeting PTEN protects against coronary microembolization-induced cardiomyocyte apoptosis in rats by activating the PI3K/AKT pathway. Eur J Pharmacol 855:244–251. https://doi.org/10.1016/j.ejphar.2019.03.045
Sharma S, Liu J, Wei J, Yuan H, Zhang T, Bishopric NH (2012) Repression of miR-142 by p300 and MAPK is required for survival signalling via gp130 during adaptive hypertrophy. EMBO Mol Med 4:617–632. https://doi.org/10.1002/emmm.201200234
Zhao Z, Qu F, Liu R, Xia Y (2019) Differential expression of miR-142-3p protects cardiomyocytes from myocardial ischemia-reperfusion via TLR4/NFkB axis. J Cell Biochem. https://doi.org/10.1002/jcb.29506
Su Q, Lv X, Ye Z (2019) Ligustrazine attenuates myocardial injury induced by coronary microembolization in rats by activating the PI3K/Akt pathway. Oxid Med Cell Longev 2019:6791457. https://doi.org/10.1155/2019/6791457
Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X, Che H, Han T, Meng S, Bai Y et al (2018) LncRNA KCNQ1OT1 mediates pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem 50:1230–1244. https://doi.org/10.1159/000494576
Ye B, Chen X, Dai S, Han J, Liang X, Lin S, Cai X, Huang Z, Huang W (2019) Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther 13:975–990. https://doi.org/10.2147/DDDT.S195412
Zheng X, Zhong T, Ma Y, Wan X, Qin A, Yao B, Zou H, Song Y, Yin D (2020) Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci 242:117186. https://doi.org/10.1016/j.lfs.2019.117186
Mishra PK, Adameova A, Hill JA, Baines CP, Kang PM, Downey JM, Narula J, Takahashi M, Abbate A, Piristine HC et al (2019) Guidelines for evaluating myocardial cell death. Am J Physiol Heart Circ Physiol 317:H891–H922. https://doi.org/10.1152/ajpheart.00259.2019
Jia C, Chen H, Zhang J, Zhou K, Zhuge Y, Niu C, Qiu J, Rong X, Shi Z, Xiao J et al (2019) Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol 67:311–318. https://doi.org/10.1016/j.intimp.2018.12.028
Kologrivova I, Shtatolkina M, Suslova T, Ryabov V (2021) Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol 12:664457. https://doi.org/10.3389/fimmu.2021.664457
Liu L, Guo Y, Li Z, Wang Z (2021) Improving cardiac reprogramming for heart regeneration in translational medicine. Cells. https://doi.org/10.3390/cells10123297
Arima Y, Fukuoka H (2020) Developmental origins of health and disease theory in cardiology. J Cardiol. https://doi.org/10.1016/j.jjcc.2020.02.003
Su Q, Liu Y, Lv XW, Dai RX, Yang XH, Kong BH (2020) LncRNA TUG1 mediates ischemic myocardial injury by targeting miR-132-3p/HDAC3 axis. Am J Physiol Heart Circ Physiol 318:H332–H344. https://doi.org/10.1152/ajpheart.00444.2019
Song S, Wen Y, Tong H, Loro E, Gong Y, Liu J, Hong S, Li L, Khurana TS, Chu M et al (2019) The HDAC3 enzymatic activity regulates skeletal muscle fuel metabolism. J Mol Cell Biol 11:133–143. https://doi.org/10.1093/jmcb/mjy066
Li Y, Liu X, Rong F (2011) PUMA mediates the apoptotic signal of hypoxia/reoxygenation in cardiomyocytes through mitochondrial pathway. Shock 35:579–584. https://doi.org/10.1097/SHK.0b013e318211601a
Wu L, Xi Z, Guo R, Liu S, Yang S, Liu D, Dong S, Guo D (2013) Exogenous ARC down-regulates caspase-3 expression and inhibits apoptosis of broiler chicken cardiomyocytes exposed to hydrogen peroxide. Avian Pathol 42:32–37. https://doi.org/10.1080/03079457.2012.757289
Ke Y, Yan H, Chen L, Zhong S, Dai Y, Cai S, Pan L, Wang Y, Zhou M (2019) Apoptosis repressor with caspase recruitment domain deficiency accelerates ischemia/reperfusion (I/R)-induced acute kidney injury by suppressing inflammation and apoptosis: The role of AKT/mTOR signaling. Biomed Pharmacother 112:108681. https://doi.org/10.1016/j.biopha.2019.108681
Acknowledgements
Thanks for all the support and contributions of the participators.
Funding
This research was funded by the grants from the National Natural Science Foundation of China (Grant No. 81960079); Guangxi Natural Science Foundation (Grant No. 2020GXNSFDA238007; Grant No. 2020GXNSFFA297002; Grant No. 2020GXNSFAA297009); The Key Research and Development Program of Guangxi (Grant No. AB20159005); Guangxi BaGui Scholars Special Project; and Guangxi Health Commission Key Laboratory of Disease Proteomics Research.
Author information
Authors and Affiliations
Contributions
Yuli Xu: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing–original draft. Xiangwei Lv: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing–original draft. Ruping Cai: Data curation, Formal analysis, Investigation, Methodology. Yanling Ren: Methodology. Rixin Dai: Methodology. Shirong He: Resources. Wei Zhang: Supervision. Quanzhong Li: Methodology, Writing–original draft. Xiheng Yang: Data curation, Formal analysis. Qiang Su: Conceptualization, Formal analysis, Supervision, Funding acquisition, Validation, Project administration, Writing–review and editing. Riming Wei: Conceptualization, Supervision, Funding acquisition, Validation, Project administration, Writing–review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experiments were approved by Guilin Medical University and produced in line with the guidance for the care and use of laboratory animals issued by National Institutes of Health (NIH). All efforts had been made to minimize pain in animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuli Xu and Xiangwei Lv contributed equally to this research.
Rights and permissions
About this article
Cite this article
Xu, Y., Lv, X., Cai, R. et al. Possible implication of miR-142-3p in coronary microembolization induced myocardial injury via ATXN1L/HDAC3/NOL3 axis. J Mol Med 100, 763–780 (2022). https://doi.org/10.1007/s00109-022-02198-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02198-z