Abstract
Despite extensive research, gliomas are associated with high morbidity and mortality, mainly attributed to the rapid growth rate, excessive invasiveness, and molecular heterogeneity, as well as regenerative potential of cancer stem cells. Therefore, elucidation of the underlying molecular mechanisms and the identification of potential molecular diagnostic and prognostic biomarkers are of paramount importance. HOX transcript antisense intergenic RNA (HOTAIR) is a well-studied long noncoding RNA, playing an emerging role in tumorigenesis of several human cancers. A growing amount of preclinical and clinical evidence highlights the pro-oncogenic role of HOTAIR in gliomas, mainly attributed to the enhancement of proliferation and migration, as well as inhibition of apoptosis. In vitro and in vivo studies demonstrate that HOTAIR modulates the activity of specific transcription factors, such as MXI1, E2F1, ATF5, and ASCL1, and regulates the expression of cell cycle–associated genes along with related signaling pathways, like the Wnt/β-catenin axis. Moreover, it can interact with specific miRNAs, including miR-326, miR-141, miR-148b-3p, miR-15b, and miR-126-5p. Of importance, HOTAIR has been demonstrated to enhance angiogenesis and affect the permeability of the blood–tumor barrier, thus modulating the efficacy of chemotherapeutic agents. Herein, we provide evidence on the functional role of HOTAIR in gliomas and discuss the benefits of its targeting as a novel approach toward glioma treatment.
Similar content being viewed by others
Introduction
Gliomas are the most common primary malignant brain tumors in adults [1]. They are associated with high morbidity and mortality. The median survival of patients with glioblastoma (GBM), the most frequent and malignant type of glioma, is only about 12 months [2]. Their rapid growth rate and excessive invasiveness to surrounding brain tissue, along with the molecular heterogeneity and regenerative potential of brain cancer stem cells, contribute significantly to their ineffective total surgical resection, resistance to chemo- and radiotherapy, and high recurrence rates [1, 3]. Historically, according to the World Health Organization (WHO), gliomas have been classified into four grades of malignancy (grades I to IV) in regard to their histopathological characteristics [4]. However, the recent discovery of specific genetic and epigenetic alterations in gliomas has led to a novel molecularly oriented approach toward the diagnosis and personalized treatment, along with the incorporation of molecular markers in the updated 2016 WHO Classification of Central Nervous System (CNS) tumors [5]. In this context, the identification of distinct molecular biomarkers and their related pathways could significantly help us toward improved diagnosis, prognosis, and effective therapeutic strategies in gliomas [6].
Only 2% of the human genome consists of protein-coding transcripts, highlighting the significance of noncoding genes [7]. There are several types of noncoding RNAs (ncRNAs) that according to their nucleotide sequence length are classified into two main categories, small and long ncRNAs. Small ncRNAs include microRNAs (miRNAs), small interfering RNAs (siRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), Piwi-interacting RNAs (piRNAs), and other short RNAs [8].
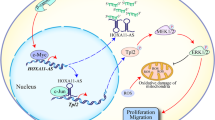
Long ncRNAs (lncRNAs) are polyadenylated RNAs composed of more than 200 nucleotides and are involved in transcriptional and posttranscriptional regulation, through binding to DNA, RNA, and proteins [9]. LncRNAs modulate the expression of numerous genes implicated in cell cycle control, cell growth, differentiation, and apoptosis in a highly tissue- and cell subtype-specific manner [10]. Furthermore, lncRNAs can act as scaffolds for various proteins in a subcellular compartment and guide them to specific target genes [11]. It has been estimated that there are about 20,000 lncRNAs in humans [12]. Not surprisingly, accumulating evidence highlights the critical multifunctional role of specific lncRNAs in several human cancers, including gliomas, by mediating pro-oncogenic or tumor-suppressive activities. For instance, antisense ncRNA in the INK4 locus (ANRIL), a lncRNA, promoted glioma development by enhancing cell proliferation, migration, and invasion, as well as inhibiting apoptosis by targeting miR-34a [13]. Additionally, the lncRNA nuclear enriched abundant transcript 1 (NEAT1) has been demonstrated to promote the invasiveness and migration, as well as suppress apoptosis of glioma cells via its implication in the miR-449b-5p/c-Met axis [14]. A different lncRNA, Maternally Expressed Gene 3 (MEG3), has been found downregulated in glioma tissues, while its overexpression enhanced apoptosis via its interaction with p53 [15]. MEG3 has been also shown to suppress glioma cell proliferation, migration, and invasion by binding to miR-19a [16], as well as to inhibit epithelial–mesenchymal transition by acting as a sponge for miR-6088 [17]. Overexpression of another lncRNA, the tumor suppressor candidate 7 (TUSC7), was demonstrated to inhibit viability, migration, and invasion of glioma cells [18]. The expression levels of TUSC7 were negatively associated with glioma grades and was suggested as a prognostic biomarker for glioma patients [18]. The lncRNA HOX transcript antisense intergenic RNA (HOTAIR) was the first trans-acting lncRNA gene to be identified and the most extensively studied [19]. It belongs to the homeobox superfamily and plays an important role in the genome-wide reprogramming of chromatin [20]. It consists of 2158 nucleotides in a single strand and is transcribed in an antisense manner from the HOXC locus on chromosome 12q13.13, one of the chromosomal loci of the clustered HOX genes (HOXA, B, C, and D) [20]. Mechanistically, its 5′ domain binds to chromatin-modifying complexes, such as polycomb-repressive complex 2 (PRC2), which contains the enhancer of zeste homolog 2 (EZH2), the suppressor of zeste homolog 12 (SUZ12), and the embryonic ectoderm development (EED) proteins (Fig. 1) [21]. The PRC2 complex induces the trimethylation of lysine 27 on histone H3 (H3K27me3), thereby suppressing the expression of various target genes [21]. The 3′ domain binds to the histone deacetylase 1 (HDAC1)/lysine-specific demethylase 1 (LSD1)/REST corepressor 1 (CoREST1)/RE1-silencing transcription factor (REST) complex, resulting in the removal of dimethylation of histone 3 lysine 4 (H3K4me2), leading to gene silencing [22].
Pro-oncogenic role of HOTAIR in gliomas. The 5′ domain of HOTAIR binds to the PRC2 complex, containing EZH2, SUZ12, and EED. The PRC2 complex induces H3K27me3, thereby inhibiting the expression of various target genes. The 3′ domain binds to the LSD1/CoREST/REST complex, resulting in the removal of H3K4me2, leading to gene silencing. The target genes of HOTAIR involve NLK, PDCD4, CDK2, CDK4, E2F1, p21, p16, CDC6, NCAPG, PLK4, CENPE, NUSAP1, NCAPH, ASPM, CEP55, and KIF4A among others. In this way, HOTAIR plays a pro-oncogenic role in gliomagenesis, promoting glioma cell proliferation, migration, invasion, and epithelial–mesenchymal transition, as well as the tumorigenicity of GSCs. HOTAIR also inhibits glioma cell apoptosis and cell cycle G0/G1 arrest. EED, embryonic ectoderm development; EZH2, enhancer of zeste homolog 2; CoREST1, REST corepressor 1; GSCs, glioma stem cells; H3K27me3, trimethylation of lysine 27 on histone H3; H3K4me2, dimethylation of histone 3 lysine 4; HDAC1, histone deacetylase 1; HOTAIR, HOX transcript antisense intergenic RNA; LSD1, lysine-specific demethylase 1; PRC2, polycomb-repressive complex 2; SUZ12, suppressor of zeste homolog 12; REST, RE1-silencing transcription factor
HOTAIR is a key regulator of chromatin dynamics. It can trans act on various genetic sites and regulate the expression of various genes [23]. HOTAIR can interact with DNA, RNA, and proteins, thereby participating in multiple cellular functions. During mammalian embryogenesis, HOTAIR is expressed in the genital bud and tail, in the hindlimb bud and posterior trunk, and in the forelimb and wrist [24]. In humans, HOTAIR is highly expressed in the skin and genital system, mainly involving the testis, prostate, and endometrium [24]. HOTAIR has been demonstrated to downregulate BMP2 and ALPL, two osteogenic-related genes, as well as inactivate various calcification-related genes, suggesting its negative regulatory role in osteogenesis [25]. In the cytoplasm, HOTAIR can interact with Mex3b and Dzip3, two E3 ubiquitin ligases, thereby being implicated in ubiquitin-mediated proteolysis and potentially in cell senescence [26]. HOTAIR also plays a significant role in cell cycle progression and proliferation, by regulating the expression of cell cycle-dependent kinase 2 (CDK2), CDK4, cyclin D1, and cyclin E [24]. However, the exact physiological role of HOTAIR has not been clarified yet.
HOTAIR is implicated in the development of various human malignancies and also serves as a promising predictive and prognostic biomarker. For instance, upregulation of HOTAIR enhances tumor growth and the metastatic potential of renal cell and hepatocellular carcinoma by inhibiting apoptosis and enhancing cell invasiveness [27, 28]. Circulating HOTAIR levels have been associated with poorer response to neoadjuvant chemotherapy and reduced survival rates of patients with breast cancer [29]. Furthermore, HOTAIR expression has been also identified as an independent prognostic factor of recurrence for urothelial carcinoma [30].
A recent study that analyzed the expression profiles of lncRNAs in brain tumor cells revealed that HOTAIR was one of the most significantly upregulated lncRNA in GBM cell lines in comparison to control human parental brain cancer stem cells [31], suggesting its pathogenic and clinical significance in gliomagenesis.
Although the role of lncRNAs in gliomas has been extensively summarized in the literature [32,33,34,35], there is no recent focused review on the role of HOTAIR in this type of cancer. Herein, we will summarize and provide an update of the growing amount of preclinical and clinical evidence highlighting the emerging role of HOTAIR-mediated pathways in glioma development and progression, as well as its diagnostic, prognostic, and therapeutic implications, aiming to shed more light on glioma pathogenesis and future research.
The pro-oncogenic role of HOTAIR in glioma development and progression: a molecular crosstalk
HOTAIR has been shown to exert a pro-oncogenic role in various types of solid tumors, such as breast cancer [36], gastric cancer [37], and renal carcinoma [38], by promoting cell proliferation, inhibiting apoptosis, and regulating cell cycle progression, as well as enhancing cell invasiveness and migration. In the following sections, we will discuss the molecular mechanisms that have been recently demonstrated to underlie the pro-oncogenic role of HOTAIR in gliomas (Fig. 1, Table 1).
Potential upstream regulatory mechanisms of HOTAIR in human gliomas
HOTAIR expression was demonstrated to be significantly elevated (up to about 30-fold) in human glioma tissues, as well as in U87 and U251 glioma cell lines, in comparison to the surrounding non-neoplastic tissues and normal brain [39, 44]. Furthermore, HOTAIR mRNA levels were significantly increased in A172 glioma cells compared to HA1800 normal astrocytes [45]. Importantly, a recent study that included data from various independent datasets demonstrated that HOTAIR was overexpressed in human high-grade glioma tissues, and particularly GBM [54].
Regarding the underlying molecular mechanisms, it was shown that the copy number aberrations in HOTAIR locus were relatively rare and they did not correlate with its expression levels [54]. On the contrary, regulation of DNA methylation levels in GBM cells by 5-azacytidine (a demethylating agent) revealed that intragenic CpGs in HOTAIR locus modulated its expression levels in a cell line-dependent manner [54]. In accordance, a study that aimed to investigate the methylation profiles of genes encoding lncRNAs in glioma tissues from The Cancer Genome Atlas (TCGA) and Cancer Cell Line Encyclopedia (CCLE) databases demonstrated that most lncRNAs were hypomethylated during glioma progression from grades II to IV, whereas HOTAIR was found to be hypermethylated, with its expression levels being positively correlated with glioma progression [61]. Moreover, the level of DNA methylation of the intergenic CpG island of HOTAIR locus has been positively correlated with HOTAIR expression in breast cancer patients [70], highlighting the important role of the methylation status of intergenic CpGs in HOTAIR overexpression in cancer, including gliomas.
Several transcription factors have been illustrated to regulate HOTAIR gene expression in tumorigenesis [71]. The oncoprotein c-Myc can directly interact with an E-box element in the upstream region of HOTAIR, thereby increasing its expression; osteopontin, an extracellular matrix protein, may also upregulate the transcription of HOTAIR, whereas interferon regulatory factor 1 (IRF1) can downregulate HOTAIR expression by binding to its promoter [71]. In breast cancer cells, estrogen receptors (ERs) and ER coregulators, such as MLL-histone methylases, can bind to the HOTAIR promoter estrogen-response-elements (EREs) in the presence of bisphenol-A or diethylstilbestrol, thereby stimulating its transcription [72]. In addition, transforming growth factor beta (TGF-β) [73] and type I collagen (Col-1) [74] can also upregulate HOTAIR expression in various cancer cell types, although the exact underlying mechanism has not been clarified yet [71].
HOXA9 was shown to directly bind to the promoter region of HOTAIR and stimulate its transcription in gliomas [54]. Given the fact that HOXA9 is regulated by the PI3K pathway through the inhibition of EZH2-mediated H3K27me3 in GBM [75] and that HOTAIR can regulate gene transcription in an EZH2-dependent manner, it was hypothesized that it may alter HOXA9 expression, thus creating a loop mechanism [54]. HOXA9 upregulation has been associated with increased glioma aggressiveness, by promoting cell viability, stemness, and invasion, as well as inhibiting apoptosis [76]. Moreover, it has been revealed that bromodomain containing 4 (BRD4) is able to directly bind to the promoter of HOTAIR gene in GBM cell lines and upregulate its expression, contributing to glioma progression [40].
Collectively, HOTAIR is overexpressed in gliomas, possibly through DNA methylation. In addition, BRD4 and HOXA9 may promote HOTAIR expression at the transcriptional level, and HOTAIR may in turn regulate HOXA9 expression, thus generating a mechanistic loop. Furthermore, given the well-established role of c-Myc and TGF-β in gliomas [77, 78], the upstream regulation of HOTAIR by these factors should be also investigated in gliomas.
HOTAIR regulation of the cell cycle–associated gene expression
A recent study reported that HOTAIR was implicated in the dysregulation of 513 molecules in GBM, including 71 transcription factors and 421 target genes [46]. It was also shown that only about 6% of these target genes were located on the chromosome where HOTAIR is found, indicating that it possibly affects the transcription of genes located in a relatively long distance of the genome (Figs. 2 and 3) [46].
HOTAIR exerts pro-oncogenic effects in gliomas via its interaction with specific transcription factors and related axes, including MXI1/CD58, MXI1/PRKCE, MXI1/CD97, E2F1/SP100, ATF5/NCAM1, ATF5/APC, ATF5/CD300A, ATF5/ADAM22, ASCL1/ARVCF, ASCL1/LRP6, and RUNX1/LSAMP pathways. Furthermore, HOTAIR can regulate several miR-related pathways, such as miR-326/FGF1, miR-141/SKA2, miR-148b-3p, miR-15b/p53, miR-126-5p/glutaminase, and miR-125a/mTOR, thereby affecting glioma development and progression. ADAM22, disintegrin and metalloproteinase domain-containing protein 22; APC, adenomatous polyposis coli; ASCL1, Achaete-scute homolog 1; LRP6, low-density lipoprotein receptor-related protein 6; HOTAIR, HOX transcript antisense intergenic RNA; LSAMP, limbic system–associated membrane protein; MXI1, MAX-interacting protein 1; mTOR, mammalian target of rapamycin; NCAM1, neural cell adhesion molecule 1; PRKCE, protein kinase C epsilon type; RUNX1, runt-related transcription factor 1
HOTAIR-related pathways in gliomas with upstream and downstream molecular factors. The oncoprotein c-Myc can directly interact with an E-box element in the upstream region of HOTAIR, thereby increasing its expression; osteopontin may also upregulate the transcription of HOTAIR, while IRF1 can downregulate HOTAIR expression by binding to its promoter. TGF-β and type I collagen (Col-1) can also upregulate HOTAIR expression at the transcriptional level in various types of cancer cells, HOXA9 can directly bind to the promoter region of HOTAIR and stimulate its transcription in gliomas. HOTAIR can suppress the transcription of NLK resulting in the positive regulation of Wnt/β-catenin pathway. Upregulation of β-catenin promotes the interaction of β-catenin with TCF/LEF transcription factors, leading to the activation of various target pro-oncogenic genes, including STAT3. NLK can phosphorylate TCF/LEF and block the interaction between the β-catenin/TCF/LEF complex and its target DNA sequence. HOTAIR inhibition is accompanied by higher E-cadherin and lower N-cadherin levels. HOTAIR inhibition is associated with reduced ZEB1, NF-κB, and twist1 expression in glioblastoma cells. The downregulation of PDCD4 in gliomas is mediated by HOTAIR. HOTAIR regulates the activity of the transcription factor (TF) MXI1 on CD58 and PRKCE; the activity of the TF ATF5 on NCAM1, APC, CD300A disintegrin, and ADAM22; the activity of the TF ASCL1 on ARVCF and LRP6; the activity of the TF E2F1 on SP100; and the activity of the TF RUNX1 on LSAMP. HOTAIR/miR-141/SKA2, HOTAIR/miR-148b-3p/USF1, HOTAIR/miR-15b/p53, HOTAIR/miR-126-5p/glutaminase, HOTAIR/miR-125a/mTOR, HOTAIR/miR-219, and HOTAIR/SNORD76 pathways are also implicated in glioma development and progression. HOTAIR, HOX transcript antisense intergenic RNA; ASCL1, Achaete-scute homolog 1; RUNX1, runt-related transcription factor 1; NCAM1, neural cell adhesion molecule 1; LRP6, lipoprotein receptor-related protein 6; SKA2, spindle and kinetochore–associated complex subunit 2; ADAM22, metalloproteinase domain-containing protein 22; PDCD4, programmed cell death protein 4; PRKCE, protein kinase C epsilon type; CD300A, cluster of differentiation 300A; STAT3, signal transducer and activator of transcription 3
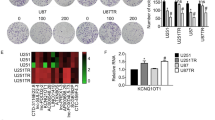
Knockdown of HOTAIR via sh-HOTAIR suppressed cell proliferation, enhanced apoptosis, and led to cell cycle G0/G1 arrest in vitro [39]. Regarding its specific target genes and the underlying molecular mechanisms, gene set enrichment analysis (GSEA) of human glioma samples indicated that HOTAIR regulates hundreds of genes mainly involved in cell cycle progression [79]. HOTAIR knockdown via si-HOTAIR treatment significantly inhibited colony formation and induced cell cycle G0/G1 arrest in LN229 and U87 cell lines, that was accompanied by reduced expression of cyclin D1, cyclin E, cyclin-dependent kinase (CDK)2, CDK4, and E2F1, as well as increased expression of the cell cycle–associated proteins p21 and p16 [79]. It has been shown that the 18 genes that were more strongly correlated with HOTAIR expression in human glioma samples obtained from the Chinese Glioma Genome Atlas (CGGA) were associated with cell cycle regulation [49]. Among them, CDC6, NCAPG, PLK4, and CENPE were the most significantly associated with HOTAIR levels in this study [49]. Abnormal cell cycle control plays a crucial role in tumorigenesis and is accompanied by aberrant expression of cell cycle checkpoint genes [80]. When entering mitosis, cells normally check the chromosomal integrity via CHEK1, CCNA2, and CCNB2 regulatory genes [81]. During mitosis, NUSAP1, NCAPH, ASPM, CENPE, CEP55, and KIF4A contribute to the regulation of the mitotic spindle and the overall mitotic process [49, 82]. The expression of these cell cycle–associated proteins has been associated with HOTAIR levels in the abovementioned study, highlighting the critical role of HOTAIR in regulating cell cycle during gliomagenesis [49].
Notably, HOTAIR enhanced cell cycle progression in LN229 and U87 GBM cell lines via an EZH2-dependent and LSD1-independent manner, indicating the critical contribution of the 5′ domain of HOTAIR to its pro-oncogenic effects in gliomas [41, 42]. EZH2 is the catalytic subunit of PRC2, which acts as a methyltransferase by adding three methyl groups to H3K27, and results in chromatin condensation and gene silencing [83]. EZH2 overexpression has been observed in GBM samples and glioma stem-like cells [84, 85], while EZH2 inhibition has been shown to induce cell cycle arrest at G0/G1 phase of glioma cells [86]. Furthermore, EZH2 phosphorylation is able to activate STAT3 (signal transducer and activator of transcription 3) pathway through STAT3 methylation, inducing a pro-oncogenic effect of GBM stem-like cells [87]. In addition, EZH2 phosphorylation can enhance the self-renewal of glioma stem-like cells via NF-κB methylation [88]. Therefore, the interaction of EZH2 with the 5′ domain of HOTAIR seems to play a pivotal role in glioma cell proliferation, enhancing tumor growth.
HOTAIR regulation of the Wnt/β-catenin pathway
HOTAIR has been shown to suppress the transcription of nemo-like kinase (NLK) in an EZH2-dependent manner in U87 cells, resulting in the positive regulation of the Wnt/β-catenin pathway, thereby inhibiting cell cycle arrest and enhancing cell migration [42]. Upregulation of β-catenin signaling is highly implicated in glioma progression. It promotes the interaction of β-catenin with TCF/LEF transcription factors, leading to the activation of various target pro-oncogenic genes, including STAT3 [89, 90]. NLK can phosphorylate TCF/LEF and block the interaction between the β-catenin/TCF/LEF complex and its target DNA sequence, acting as a negative regulator of β-catenin pathway [91]. Notably, the effects of HOTAIR on NLK transcription were independent of the epidermal growth factor receptor (EGFR) activation status of U87 cells [42]. Abnormal overactivation of EGFR signaling has been associated with GBM progression and prognosis [92, 93], and the amplification of the EGFR gene is a common genetic mutation in GBM [93]. Interestingly, HOTAIR inhibition was accompanied by higher E-cadherin and lower N-cadherin levels in these cells, consistent with the participation of this lncRNA in the epithelial–mesenchymal transition (EMT), a process that drives cell invasion and migration in many cancers including gliomas [42]. The expression of E-cadherin is known to be directly or indirectly regulated by various transcription factors, such as ZEB1 [94], NF-κB [95], and twist1 [96]. Interestingly, HOTAIR inhibition was associated with reduced ZEB1, NF-κB, and twist1 expression in GBM cells, confirming the significant role of HOTAIR in glioma cell invasiveness. Importantly, the Wnt/β-catenin pathway has been shown to play an important role in the EMT process [97], and the reduced expression of E-cadherin has been associated with increased expression of metalloproteinases (MMPs) in gliomas [46]. In accordance, HOTAIR expression levels were found positively correlated with MMP-7 and MMP-9 expression in human glioma specimens [63]. Therefore, HOTAIR seems to promote glioma progression by stimulating the Wnt/β-catenin pathway, thus resulting in enhancement of glioma cell proliferation and invasiveness.
Of note, it has been shown that the lncRNA papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) is able to inhibit the proliferation, migration, and epithelial–mesenchymal transition of glioma cells by downregulating the Wnt/β-catenin axis [98]. A recent study indicated that PTCSC3 overexpression inhibited HOTAIR by downregulating STAT3, thus suppressing the proliferation of laryngeal squamous cell carcinoma cells in vitro [99]. In addition, STAT3 was found to promote HOTAIR expression in this study. Given the well-known significance of the Wnt/β-catenin/STAT3 pathway in gliomas [90], the role of PTCSC3 as a potential upstream inhibitor of HOTAIR expression via STAT3 should be further investigated in gliomas and may possibly serve as a pharmaceutical target.
HOTAIR targeting of PDCD4
HOTAIR has been also shown to interact with the programmed cell death protein 4 (PDCD4) which was demonstrated to suppress cell proliferation and migration in U251 glioma cells that express lower levels compared with normal astrocytes [44]. The downregulation of PDCD4 in gliomas was related to increased H3K27me3 at the promoter region of PDCD4 gene, and mediated by HOTAIR in a PRC2-dependent manner [44]. Lower PDCD4 expression has been associated with higher chemotherapy resistance in GBM by inhibiting the translation of B cell lymphoma-extra-large (Bcl-xL), a key anti-apoptotic factor [100]. Furthermore, PDCD4 downregulation by miR-21 could promote glioma cell proliferation in vivo [101]. Hence, epigenetic inhibition of PDCD4 gene expression is another mechanism via which HOTAIR enhances glioma cell proliferation and migration, as well as promotes an anti-apoptotic/survival phenotype.
Although under normal conditions stem and progenitor cells contribute to tissue repair and development, a small percentage of cells (0.1–1%) from human solid tumors, including gliomas, display stem-like properties and play a critical role in the resistance to chemotherapy and radiotherapy, as well as to tumor recurrence [102]. A recent study showed that HOTAIR was highly expressed in glioma stem cells (GSCs) expressing CD133 (the most widely used marker of GSCs) isolated from human glioma specimens, compared with the CD133− glioma cells [47]. Inhibition of the expression of HOTAIR by superparamagnetic iron oxide nanoparticles (SPION)-mediated si-HOTAIR transfection significantly decreased the proliferation, migratory capacity, and tumorigenicity of GSCs [47]. This effect was mediated by the si-HOTAIR-mediated enhanced PDCD4 expression at the transcriptional level [47]. In particular, HOTAIR was shown to modulate PDCD4 transcription by affecting the recruitment of EZH2 and LSD1 at the promoter region of PDCD4, leading to reduced H3K27me3 and increased H3K4me2 levels [47]. Hence, HOTAIR seems to regulate the interaction of histones with the promoter region of PDCD4 in GSCs, resulting in tumor promotion [47].
HOTAIR/MXI1 pathway
Emerging evidence highlights the capacity of lncRNAs to affect the activity of transcription factors, which are key regulatory components of gene expression in cancer [103]. In this context, a recent study that used a computational method combining lncRNA and gene expression profiles with transcription factor–target correlations in GBM samples indicated that 12 HOTAIR/transcription factor/gene triplets were associated with overall survival of glioblastoma patients, including HOTAIR/MAX-interacting protein 1 (MXI1)/CD58, HOTAIR/MXI1/protein kinase C epsilon type (PRKCE), and HOTAIR/MXI1/CD97 [46]. In particular, HOTAIR was shown to invert the activity of the transcription factor MXI1 on CD58 and PRKCE [46]. Overexpressed MXI1 has been already indicated to suppress U87 GBM cell proliferation via downregulation of cyclin B1 gene expression [104]. CD58 could upregulate the Wnt pathway that is highly implicated in glioma progression [105], whereas PRKCE, one of the 11 isoenzymes of protein kinase C (PKC), has been shown to be overexpressed in astroglial cell lines and glioblastoma samples [106]. Hence, it can be speculated that HOTAIR may inhibit the MXI1-induced downregulation of CD58 and PRKCE gene expression, thus enhancing their expression and subsequently their pro-oncogenic effects. It has been also demonstrated that CD97 can promote glioma cell invasion and migration and is thus associated to poorer prognosis of GBM patients [107]. Collectively, HOTAIR can regulate the activity of MXI1, thus affecting critical pathways in glioma development, at least partially via CD58, PRKCE, and CD97.
HOTAIR/ATF5 pathway
In the abovementioned study, HOTAIR was demonstrated to regulate the activity of the activating transcription factor 5 (ATF5) on neural cell adhesion molecule 1 (NCAM1), adenomatous polyposis coli (APC), cluster of differentiation 300A (CD300A), disintegrin, and metalloproteinase domain-containing protein 22 (ADAM22) [46]. ATF5 upregulation contributes essentially to gliomagenesis [108], and the Wnt/β-catenin pathway has been shown to downregulate the tumor-suppressive activity of NCAM1 in glioma cells, thus promoting glioma progression [109]. ADAM22 is a brain-specific cell surface protein capable to inhibit glioma cell proliferation via an integrin-dependent pathway [110]. However, the exact effects of the interaction between HOTAIR and the TFs in the expression of these genes, as well as the subsequent effects on cellular function, are still unclear and remain to be elucidated.
HOTAIR/ASCL1 pathway
Two other triplets identified in the abovementioned experiment are HOTAIR/Achaete-scute homolog 1 (ASCL1)/ARVCF and HOTAIR/ASCL1/low-density lipoprotein receptor-related protein 6 (LRP6) [46]. ASCL1 is a proneural transcription factor implicated in normal neurogenesis and has been shown to play a significant role in gliomas by reorganizing chromatin and suppressing tumorigenicity of GBM stem cells [111]. LRP6 has been shown to be overexpressed in gliomas and may act as an upstream regulator of glycogen synthase kinase-3β (GSK3β) pathway [112], while ARVCF expression has been demonstrated to contribute to the tumor-suppressive function of p53 [113]. The specific mechanism of HOTAIR/ASCL1/ARVCF and LSP6 interaction is still unclear, but HOTAIR can be speculated to inhibit ASCL1, thereby allowing for ARVCF and LRP6 down- and upregulation, respectively.
HOTAIR/E2F1/SP100 pathway
HOTAIR/E2F1/SP100 has been also shown to be implicated in glioma development [46]. E2F1 has been involved in glioma cell proliferation [114], while SP100 has been reported to suppress malignant behavior of glioma cells [115]. In particular, HOTAIR may target and inhibit the transcription factor E2F1, resulting in the downregulation of SP100 expression.
HOTAIR/RUNX1/LSAMP pathway
Runt-related transcription factor 1 (RUNX1) is also a transcription factor whose activity on LSAMP was mediated by HOTAIR [46]. RUNX1 has been shown to contribute to the mesenchymal subtype of GBM through a TGFβ-dependent pathway [64], and RUNX1 overexpression has been illustrated to reduce glioma growth via downregulation of pro-oncogenic genes [116]. Although the exact mechanism has not been clarified yet, it can be speculated that HOTAIR may target and inhibit RUNX1, thus allowing LSAMP gene expression.
Therefore, HOTAIR may regulate the activity of these transcription factors on various genes, affecting crucial signaling pathways implicated in gliomagenesis and progression.
HOTAIR/miR-326/FGF1 pathway
Of note, the interaction between lncRNAs and miRNAs has been increasingly recognized as an additional regulatory process of gene expression (Fig. 2) [117]. LncRNAs may act as competing endogenous RNAs (ceRNAs) or molecular sponges of miRNAs, by interacting and competing with them for binding to target mRNAs [118]. The pro-oncogenic properties of HOTAIR have been mediated via the downregulation of specific miRNAs, including miR-7 in breast cancer [119], miR-130a in gallbladder cancer [52], and miR-331-3p in gastric cancer [118].
In this context, HOTAIR knockdown via sh-HOTAIR upregulated miR-326 in U87 and U251 glioma cells, resulting in the inhibition of fibroblast growth factor 1 (FGF1) overexpression at both the mRNA and protein levels [39]. These events were accompanied by reduced cell proliferation, increased apoptosis, and cell cycle arrest in G0/G1 phase, as well as decreased cell migration and invasion [39]. Overexpression of FGF1 was also shown to activate phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and mitogen-activated protein kinase kinase (MEK)1/2 signaling pathways in this study [39]. miR-326 has been previously shown to be downregulated in gliomas [120], and FGF1/fibroblast growth factor receptor (FGFR) signaling can sustain the stem cell characteristics of GBM cells [121]. PI3K/AKT and MEK1/2 pathways have been shown to be critical mediators of the pro-oncogenic properties of FGFR activation in esophageal squamous cell carcinoma [122] and endometrial cancer [123] and are also highly implicated in GBM [124]. Collectively, the HOTAIR/miR-326/FGF1 axis seems to play a pivotal role in glioma cell proliferation, apoptosis, and invasion, and PI3K/AKT and MEK1/2 pathways represent potential signaling mediators of this process [39].
HOTAIR/miR-141/SKA2 pathway
Growing evidence indicates that miRNAs can themselves regulate the expression of lncRNAs. For instance, miR-34a is able to bind to HOTAIR and reduce its expression levels in prostate cancer [125], while miR-141 can target HOTAIR in renal cell carcinoma [126]. It was recently revealed that miR-141 was able to directly bind to the 3′ UTR of HOTAIR in U251 and U87 glioma cells, inhibiting its expression [48]. miR-141 suppressed glioma cell proliferation and invasion in this study by interacting with spindle and kinetochore–associated complex subunit 2 (SKA2), while HOTAIR reversed these effects [48]. Importantly, luciferase assays revealed the specific molecular crosstalk between HOTAIR and SKA2 in the competition for binding to miR-141 [48]. SKA2 is implicated in the maintenance of the metaphase and spindle checkpoint silencing [127] and may play a pro-oncogenic role in U251 glioma cells by promoting cell proliferation and migration [128].
HOTAIR/miR-148b-3p pathway
In the same context, a recent study confirmed the pro-oncogenic role of HOTAIR in A172 glioma cells, where it promoted cell proliferation, migration, and cell cycle progression [45]. The reciprocal repression of HOTAIR and miRNA-148b was revealed as the underlying mechanism, given the fact that miR-148b-3p directly targeted HOTAIR gene sequence, thus inhibiting its expression. Knockdown of HOTAIR via si-HOTAIR upregulated miR-148b-3p [45]. Thus, miR-148b-3p possibly suppressed the malignant behavior of glioma cells in vitro by reducing the expression of HOTAIR, whereas HOTAIR could inversely reduce miR-148b-3p expression [45]. Another recent study indicated that HOTAIR/miR-148b-3p/upstream stimulating factor 1 (USF1) pathway may affect the permeability of the blood–tumor barrier in gliomas [50]. miR-148b-3p is implicated in various types of tumors acting as a tumor-suppressive or tumor-promoting factor, such as in pancreatic [129] and ovarian cancer [130], respectively. Although its role in gliomas has not been elucidated yet, this study highlights the importance of HOTAIR/miR-148b-3p interaction in glioma development that needs to be further investigated.
HOTAIR/miR-15b/p53 pathway
A recent study demonstrated that HOTAIR may act as a pro-oncogenic factor in human U87 glioma cells by reducing miR-15b expression [55]. In particular, miR-15b could increase p53 expression, thus inhibiting proliferation and invasion, as well as promoting apoptosis of glioma cells [55]. HOTAIR upregulation was able to suppress the tumor-suppressive properties of miR-15b and p53 in this experiment [55]. Inversely, miR-15b decreased the expression of HOTAIR via p53 [55].
Interestingly, miR-15b has been shown to be downregulated in GBM specimens and cells, while its levels were found to be associated with patients’ survival [131]. miR-15b mimics were demonstrated to inhibit the proliferation and invasion of U87 and U251 cells, by targeting the insulin-like growth factor receptor-1 (IGF1R) [131]. The tumor-suppressive role of the transcription factor p53 is already well described in many tumors including gliomas, since the dysregulation of p53-related axis has been implicated in GBM cell proliferation, invasion, migration, and apoptosis, as well as glioma cell stemness [132]. Taken together, HOTAIR, miR-15b, and p53 seem to form a regulatory loop affecting glioma progression, constituting another significant molecular pathway underlying the tumor-promoting role of HOTAIR in gliomas.
HOTAIR/miR-126-5p/glutaminase pathway
HOTAIR/miR-126-5p/glutaminase has been recently demonstrated as another pathway underlying HOTAIR-mediated gliomagenesis and progression [56]. HOTAIR was found to enhance the malignant behavior of U87 and U251 glioma cells by acting as a ceRNA that binds competitively to miR-126-5p. Through this mechanism, it was shown to promote cell invasion, modulate glutamine metabolism, increase angiogenesis, and reduce the chemosensitivity to temozolamide [56]. HOTAIR inhibition was shown to reduce the expression of endogenous glutaminase, a crucial enzyme for glutamine catabolism, leading to reduced glutamate and glutathione (GSH) levels in these cell lines [56]. Glutaminase isoforms have been indicated to diversely affect glioma cell [133]. In particular, kidney-type glutaminase isoforms (KGA and GAC) encoded by the GLS gene, which is regulated by the oncogene c-Myc, enhance cell proliferation, whereas liver-type isoforms (GAB and LGA) encoded by the GLS2 gene inhibit cell proliferation and migration and promote the sensitization of glioma cells to chemotherapeutic alkylating agents [133]. Furthermore, glutaminase inhibitors have been shown to reduce therapy resistance of human high-grade gliomas in vitro [134]. Hence, HOTAIR seems to represent an important player in the regulation of glutaminase in gliomas, although its exact effects on the specific glutaminase isoforms remain to be elucidated. miR-126 has been already indicated to act as a tumor suppressor in gliomas by negatively regulating insulin receptor substrate 1 (IRS-1) [135] and inhibiting extracellular signal-regulated kinase (ERK) axis through downregulating kirsten rat sarcoma viral oncogene (KRAS) [136]. Therefore, HOTAIR may represent one of the upstream regulators of miR-126 in gliomas affecting cell invasiveness.
HOTAIR/miR-125a/mTOR pathway
Schisandrin B, derived from the Chinese medicinal herb Schisandra chinensis Baill, has been indicated to exert antitumor properties in some human cancers including gliomas, by suppressing glioma cell proliferation and invasion [137]. A recent study demonstrated that Schisandrin B reduced HOTAIR expression and enhanced miR-125a-5p expression in U251 and U87 glioma cell lines by downregulating the mammalian target of rapamycin (mTOR) expression, resulting in decreased cell viability, migration, and invasion, as well as increased apoptosis [51]. miR-125a-5p has been shown to suppress glioblastoma cell proliferation and invasion [138], and HOTAIR has been demonstrated to inhibit miR-125a expression [139]. mTOR is a crucial downstream mediator of the PI3K/AKT pathway, which plays a pivotal role in many cancer types including gliomas [137]. Therefore, Schisandrin B has been shown to display inhibitory effects on glioma progression in vitro, via the HOTAIR/miR125a/mTOR pathway.
HOTAIR/miR-219 pathway
HOTAIR has been also shown to target and inhibit miR-219 in U87 GBM cell line, resulting in increased cell proliferation and cyclin D1 levels, as well as reduced apoptosis and Bax expression levels [69]. miR-219-5p acts as a tumor suppressor factor in gliomas by targeting EGFR [140]. Since HOTAIR restores gefitinib sensitivity by stimulating Bax/caspase-3 and inhibiting the TGF-α/EGFR signaling pathway in lung adenocarcinoma [57], this mechanism should be explored in gliomas, too.
HOTAIR/SNORD76 pathway
HOTAIR has been also demonstrated to regulate the expression of small ncRNAs in solid tumors. In particular, HOTAIR overexpression has been shown to inhibit the expression of a C/D box snoRNA U76 (SNORD76) in glioma cell lines [43]. SNORD76 is located in the third intron of the DNA sequence of GAS5 and is produced by processing GAS5 pre-RNA to GAS5 mature RNA [141]. Thus, consistent alterations of both SNORD76 and GAS5 would be expected when levels of HOTAIR are modified [43]. Given the fact that HOTAIR significantly affected SNORD76 but not GAS5 expression, it has been proposed that HOTAIR might rather act through regulation of splicing than by epigenetic silencing [43]. Forced expression of SNORD76 was shown to inhibit cell proliferation by inducing S phase cell cycle arrest of glioma cells in vitro and impede orthotopic tumor growth in vivo [43]. Hence, the regulation of the snoRNA SNORD76 expression by HOTAIR may represent another potential molecular mechanism contributing to gliomagenesis.
The role of HOTAIR in glioma development and progression: in vivo evidence
On top of in vitro evidence, the pro-oncogenic role of HOTAIR in gliomas has been also investigated in vivo. More specifically, si-HOTAIR-treated U87 orthotopic glioma mouse models demonstrated reduced tumor growth [79]. Additionally, HOTAIR inhibition via si-HOTAIR reduced tumor growth, increased apoptosis, and resulted in the longer survival of GBM xenograft mouse models, mainly mediated by its 5′ domain [41]. HOTAIR knockdown in U87 orthotopic GBM mouse models by si-HOTAIR treatment was shown to inhibit tumor growth, independent of EGFR activation status, while it was accompanied by longer survival [42]. This study also confirmed the inhibition of cell cycle of U87 cells at G1 phase in vivo. Furthermore, HOTAIR knockdown reduced tumor growth, promoted apoptosis, and increased the survival of nude mice models, while the combined miR-326 overexpression further enhanced these effects [39]. miR-141 also decreased cell proliferation and xenograft tumor growth in nude mice in vivo via its interaction with HOTAIR and the regulation of SKA2 expression [48]. In addition, SPION-mediated si-HOTAIR transfection inhibited the tumorigenicity of GSCs in the same model [47]. Importantly, a recent study demonstrated that HOTAIR knockdown decreased cell viability, inflammation, tumor growth, invasive capacity, and the number of satellite metastases, as well as increased apoptotic rates of orthotopic glioma models that were established in nude mice via inoculation of glioma cell suspension [58]. Of note, these effects were accompanied by better clinical outcomes, in the absence of convulsions and hemiplegia, as well as by longer survival in the sh-HOTAIR-treated group [58]. HOTAIR knockdown in U87vIII glioma cells suppressed mouse intracranial GBM model formation in another study [49] and decreased tumor growth in nude mice that were injected with U87 glioma cells [56].
HOTAIR enhances angiogenesis by upregulating VEGFA expression
Angiogenesis is an essential process of gliomagenesis, since the survival, proliferation, and invasion of cancer cells constantly require oxygen and nutrients that are supplied by the growth of new blood vessels [142]. Glioma cells have been shown to promote angiogenesis by triggering the expression and secretion of growth factors, including vascular endothelial growth factor (VEGF) [143]. It has been shown that HOTAIR levels positively correlate with VEGF expression in glioma tissues obtained from glioma patients, highlighting a potential association between them [63]. Indeed, a recent study demonstrated that HOTAIR could promote glioma-induced proliferation and migration of endothelial cells, as well as tube formation, by inducing VEGFA expression in A172 GBM cells [144]. Importantly, it was also shown that extracellular HOTAIR was mainly present in membranous structures in the supernatant of GBM cell culture, instead of being directly released [144]. Therefore, it has been proposed that HOTAIR may exert pro-angiogenic properties in gliomas not only by increasing VEGFA expression but also through formation of extracellular vesicles derived from glioma cells [144]. HOTAIR has been shown to increase angiogenesis in nasopharyngeal carcinoma cells by directly targeting the promoter and enhancing the transcription of VEGFA [145]. In rapidly growing gliomas, it has been shown that hypoxia may upregulate hypoxia-inducible factor 1 (HIF-1), which in turn induces VEGFA transcription [146]. In renal cell carcinoma models, HOTAIR facilitated HIF-1α expression, resulting in increased cell proliferation, migration, and decreased apoptosis by inhibiting miR-217 [147], suggesting a similar mechanism of action in gliomas. Extracellular vesicles can be released by cancer cells and affect tumor microenvironment by transferring “messages” to neighboring cells. HOTAIR has been detected in extracellular vesicles secreted by human cervical and breast cancer [148]. Therefore, HOTAIR represents another molecular mechanism underlying enhanced angiogenesis in gliomas. The translocation of HOTAIR via glioma cell-derived extracellularly released vesicles to tumor endothelial cells should therefore be further investigated.
Diagnostic and prognostic significance of HOTAIR in gliomas
Diagnostic potential of HOTAIR in gliomas
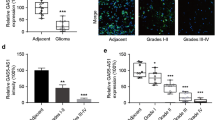
HOTAIR expression levels were higher in human glioma specimens compared to normal brain tissue [42], and serum HOTAIR levels were increased in GBM patients in comparison to controls [23]. A study employing single molecule sequencing (SMS) expression analysis showed that HOTAIR was expressed in most human GBM specimens, being absent in normal brain tissues, thus suggesting its potential specificity for gliomas [40]. However, as mentioned above, HOTAIR is highly expressed in the skin and genital system, mainly involving the testis, prostate, and endometrium, as well as during embryogenesis in specific tissues [24]. HOTAIR was upregulated in the midbrain of MTPT-induced PD mice models [149], and higher expression levels have been demonstrated in the peripheral blood mononuclear cells (PBMCs) of vitamin D-deficient patients with multiple sclerosis compared with controls [150], suggesting that HOTAIR levels may be elevated in the context of neurodegenerative or neuroinflammatory processes. Additionally, serum HOTAIR levels have been increased in patients with other tumors as compared with controls, such as esophageal squamous cell carcinoma [151]. Therefore, since HOTAIR is also expressed in other tissues and pathological conditions, significant concerns have arisen for its use as a diagnostic biomarker in gliomas, being rather indicative of an abnormal health status than specifically diagnosing glioma.
Prognostic significance of HOTAIR expression in glioma tissues
HOTAIR has been already identified as a marker of poor prognosis in breast, hepatocellular, gastric, colorectal, esophageal, and lung cancer patients [151,152,153,154,155,156]. A recent meta- and bioinformatics analysis indicated that its expression was significantly associated with reduced overall survival in several human cancers [157].
The prognostic value of HOTAIR in gliomas was firstly demonstrated in a study investigating HOTAIR expression in 295 glioma samples from the CGGA, REMBRANDT, and GSE4290 glioma datasets [79]. In particular, HOTAIR expression levels were significantly positively correlated with tumor grade, and glioma patients with high HOTAIR levels had shorter overall survival [79]. In accordance, whole gene profiling of 310 human glioma and 5 normal brain tissues from the CGGA1 database demonstrated that its expression was higher in GBM specimens in comparison to low-grade and normal tissues, as well as in patients with shorter survival [42]. Furthermore, analyses of data from glioma patients documented in the TCGA along with four additional cohorts from the Oncomine database, as well as two French and Portuguese glioma data series, demonstrated that HOTAIR expression was higher in grade III and IV glioma specimens as compared with grade II and normal brain tissues [54]. Of note, survival of patients with GBM, grade III or grade II gliomas, and higher HOTAIR expression was shorter in this study [54], highlighting its prognostic value across all tumor grades. HOTAIR levels were also increased in high-grade gliomas compared with low-grade ones, as shown in a study of 158 glioma specimens from the CGGA database [56]. Additionally, microarray analysis of six GBM and normal brain tissues combined with clinical data from the TCGA revealed that nine lncRNAs, including HOTAIR, were significantly associated with overall survival of GBM patients [59]. HOTAIR expression levels were also positively correlated with glioma grade in another study that examined specimens from 123 patients with gliomas. In this study, the HOTAIR level > 0.40 was shown to be an independent risk factor for gliomas of grades II–IV [63]. Interestingly, a recent study that used a computational method combining lncRNA and gene expression profiles with transcription factor–target correlations in GBM samples demonstrated that 12 HOTAIR/transcription factor/gene triplets were associated with overall patients’ survival [46]. Examples of these triplets were shown to involve HOTAIR/MXI1/CD58, HOTAIR/MXI1/PRKCE, HOTAIR/E2F1/SP100, HOTAIR/MXI1/CD97, HOTAIR/ATF5/APC, and HOTAIR/ATF5/NCAM1 [46]. Notably, in most cases, expression profiles of individual transcription factors were not correlated with overall survival, suggesting that the use of “triple biomarkers” may be more appropriate than individual genes in the case of glioma prognosis [46].
The role of HOTAIR in glioma molecular subclassification
Importantly, HOTAIR expression has been hypothesized to play a critical role in glioma molecular subclassification. In this context, the mesenchymal-like glioma subtype (composed of classical and mesenchymal subtypes) expressed higher levels of HOTAIR in comparison to proneural-like subtype (composed of neural or proneural subtypes) in the CGGA, REMBRANDT, and GSE4290 databases [79]. This proposed classification system has been mainly based on the response to temozolamide treatment among these distinct subtypes [158].
Furthermore, analysis of the TGCA data showed that HOTAIR expression was associated with isocitrate dehydrogenase (IDH) mutation status in grade III but not grade II gliomas, since HOTAIR levels have been demonstrated to be significantly correlated with IDH wild-type grade III glioma specimens [54]. IDH1/2 mutations are present in about 50–80% of gliomas and have been associated with a longer survival of glioma patients [159]. Therefore, the observed association between HOTAIR and the presence of IDH1/2 wild-type strengthens its pro-oncogenic and prognostic potential in gliomas that deserves further study.
However, another study failed to detect any differences between HOTAIR levels and any GBM subgroup [23], although no detailed data were provided concerning the adopted analytical approaches.
Prognostic significance of serum HOTAIR levels
Although it is still unclear how lncRNAs are released extracellularly, they can be also detected in biological fluids, including serum and urine. LncRNAs are characterized by relatively stable secondary structures which allow their usage as potential peripheral biomarkers in several cancers [23]. For instance, increased circulating HOTAIR levels have been associated with poorer prognosis of cervical cancer [160] and tumor, node, and metastasis (TNM) staging of esophageal squamous cell carcinoma [151]. In addition to the diagnostic and prognostic value of HOTAIR expression in glioma tissues, the clinical significance of its serum levels has been also investigated. A study that examined the levels of six oncogenic lncRNAs [colorectal neoplasia differentially expressed (CRNDE), MALAT1, HOTAIR, growth arrest-specific 5 (GAS5), H19, and taurine upregulated gene 1 (TUG1)] in the serum of 106 GBM patients indicated that higher HOTAIR levels were correlated with increased likelihood of progression and recurrence of the disease, as well as shorter survival [60]. In agreement with these results, another study including 43 patients with GBM, 23 patients with lower-grade gliomas, and 40 controls demonstrated that serum HOTAIR levels detected by quantitative real-time PCR (qRT-PCR) were increased in GBM patients as compared with controls or lower-grade gliomas [23]. Importantly, HOTAIR was detected in serum exosomes purified from GBM patients and not in serum supernatant depleted of exosomes [23]. Exosomes are membrane-bound small vesicles that can be secreted extracellularly by tumor cells into biological fluids [161]. It has been shown that exosomes may contain mRNAs and lncRNAs that may mediate cell-to-cell communication in gliomas [161]. Notably, serum HOTAIR expression levels were correlated with its tissue levels in 15 paired GBM tumor tissues and serum samples, implying that serum HOTAIR may be derived from the glioma tissue [23].
Importantly, a recent study including 51 GBM patients receiving temozolamide treatment [25 patients in the responding group (complete response and partial response) and 26 patients in the nonresponding group (stable disease and progressive disease)] demonstrated that HOTAIR levels in serum exosomes were higher in nonresponders compared with responders, suggesting its potential use as a biomarker for responsiveness to temozolamide in GBM patients [68].
Prognostic role of SNPs in the promoter regions of HOTAIR in gliomas
Genome-wide association studies (GWAS) have revealed specific single nucleotide polymorphisms (SNPs) in cancer-related genes that are associated with glioma susceptibility, such as EGFR, RTEL1, and TGF-β1 [162, 163]. It has been shown that the SNPs rs920778 (C > T) in the intronic enhancer and rs12826786 (C > T) in the promoter regions of HOTAIR may be associated with cancer risk and prognosis for some tumors, such as breast cancer [164, 165] and esophageal squamous cell carcinoma [166]. A study of 177 Portuguese glioma patients and 199 controls indicated that the frequencies of HOTAIR SNPs rs920778 and rs12826786 did not significantly differ between these groups, independently of WHO grade glioma [53]. Glioma patients carrying the rs12826786 CT genotype exhibited greater intratumoral HOTAIR levels, in comparison to TT but not CC genotype [53]. On the other hand, no differences were detected regarding rs920778 [53]. These results are in discrepancy with other studies, in which higher HOTAIR levels were observed in gastric cancer patients carrying the rs920778 TT [167] or rs12826786 TT genotype [168]. These differences may be explained by the highly tissue- and tumor-specific roles of lncRNAs SNPs. In the abovementioned study, HOTAIR SNPs rs920778 and rs12826786 were not associated with overall survival of GBM patients [53]. However, in a small study of patients with anaplastic oligodendroglioma, those carrying the rs920778 or rs12826786 CT genotype displayed longer overall survival, indicating the need for larger cohort studies involving this specific glioma subtype [53].
HOTAIR as a novel therapeutic target in gliomas
Specific pharmaceutic approaches have been already developed toward targeting lncRNAs in human cancers, including small molecule inhibitors, antisense oligonucleotides (ASOs), and RNA interference (RNAi) technology [169].
As discussed above, HOTAIR has been shown to regulate glioma progression via its 5′-domain in an EZH2-dependent manner. Therefore, pharmaceutical targeting of HOTAIR-EZH2 interaction by small molecules may serve as a novel treatment approach. AC1Q3QWB can inhibit glioma progression acting as a HOTAIR-EZH2 inhibitor in glioma cells expressing high levels of HOTAIR and EZH2 [62]. In addition, AC1Q3QWB suppressed the Wnt/β-catenin pathway by upregulating APC2 expression, thus reversing EMT and inhibiting cell proliferation [62]. AC1Q3QWB was able to reverse the HOTAIR-PRC2-induced epigenetic gene silencing by specifically inhibiting HOTAIR-EZH2 interaction and disrupting PRC2 recruitment [62]. In this regard, the combination of AC1Q3QWB and palbociclib, a CDK4/6 inhibitor, exerted stronger antiproliferative and antimetastatic effects on gliomas both in vitro and in vivo, compared with the single drug alone [65]. The tumor suppressor gene CWF19L1 was upregulated by AC1Q3QWB, resulting in the degradation of CDK4/6, leading to G1 arrest [65]. Thus, AC1Q3QWB may represent a promising pharmaceutical agent toward glioma treatment.
Compared to genetic mutations, epigenetic modifications are reversible, suggesting that their targeting in cancer may represent a promising treatment strategy [40]. In this context, the bromodomain and extraterminal (BET) proteins act as epigenetic regulators and they have emerged as potential pharmaceutical targets for various cancers [40]. Among BET inhibitors, I-BET151 has been shown to suppress GBM progression through unknown mechanisms. Interestingly, a recent study revealed that HOTAIR inhibition was evident upon I-BET151-induced cell cycle arrest in GBM cells [40]. More specifically, it was shown that I-BET151 could decrease the binding of BRD4 to the promoter region of HOTAIR and suppress its expression [40].
Accumulating evidence reveals that direct or indirect lncRNA targeting underlies at least partially the antitumor effects of phytochemicals. In particular, genistein and calycosin, two isoflavones, have been demonstrated to reduce HOTAIR expression and promote apoptosis of breast cancer MCF-7 cells in vitro [170]. Genistein can also inhibit HOTAIR expression and upregulate miR-141 in renal cell carcinoma [126], as well as reduce HOTAIR levels in prostate cancer cell lines [125]. Of note, genistein has shown antitumor effects in gliomas by downregulating MMP2 and VEGF expression, as well as reducing DNA synthesis [171, 172]. Silibinin, a polyphenolic flavonolignan, can decrease HOTAIR levels in bladder cancer cells [173]. In vivo evidence has revealed the pro-apoptotic and antitumor effects of silibinin in glioblastoma [174]. Therefore, HOTAIR regulation may represent another potential target of genistein and silibinin also in gliomas. These phytochemicals can target multiple proteins and regulate various pathways, including mTOR, PI3K, AKT, NF-κB, and β-catenin [169], which are also implicated in glioma development [175]. Hence, given also their minimum toxicity, these agents represent a promising therapeutic approach against gliomas, while their ability to target HOTAIR and other lncRNAs needs further investigation.
Cisplatin and paclitaxel treatment have been associated with decreased HOTAIR, CDKN2B-AS1, and MALAT1 expression levels in laryngeal cancer cell lines in a dose- and time-dependent manner [176], implying that these lncRNAs were targets of the chemotherapeutic drugs.
ASOs are short single-stranded DNAs or RNAs targeting specific RNAs, and they can modulate lncRNA function via their degradation [177]. RNAi involves the knockdown of specific genes via neutralizing RNAs, including short interfering RNAs (siRNAs) and short hairpin RNAs (shRNAs) [178]. SiRNAs are short double-stranded RNAs targeting specific complementary RNA molecules, resulting in posttranscriptional RNA silencing [177]. Concerning the use of si-HOTAIR in glioma therapy, an important aspect that needs to be considered is its effective delivery into tumor cells [179]. Recently, iron oxide nanoparticles and specifically SPIONs have attracted attention as carriers of nucleic acids, due to their high stability and susceptibility to various modifications [180]. In particular, upon binding to specific siRNAs, SPIONs can deliver the carried nucleic acids into eukaryotic cells under the influence of an external magnetic field. The si-HOTAIR has been shown to be successfully delivered via SPIONs in glioma stem cells, inhibiting glioma progression [47]. Therefore, this novel strategy may represent an effective way of transfecting glioma cells with si-HOTAIR and affect their malignant behavior.
Temozolamide is the most commonly used chemotherapeutic agent for GBM [181]; however, chemoresistance to this drug is still an unresolved obstacle contributing to tumor recurrence. In this regard, developing strategies for increasing the sensitivity of gliomas to temozolamide present a field of current research to improve prognosis. Of note, a very recent study indicated that the deletion of a HOTAIR regulatory element initially identified as a promoter of esophageal squamous cell carcinoma progression [166] enhanced the sensitivity of U251 glioma cells to temozolamide, possibly through the long-range inhibition of CALCOCO1 and ZC3H10 genes [66]. HOTAIR has been illustrated to increase the expression of hexokinase 2 by downregulating miR-125, leading to the inhibition of GBM cell proliferation and enhancement of the temozolamide-induced apoptosis [67]. HOTAIR was upregulated in temozolamide-resistant GBM cells, while exosome-mediated transfer of HOTAIR to GBM cells led to temozolamide resistance by negatively regulating miR-519a-3p and subsequently allowing RRM1 expression [68]. These findings highlight the role of HOTAIR in temozolamide sensitivity, paving the way for further research.
Because of the blood–tumor barrier (BTB), the use of macromolecular chemotherapeutic drugs in gliomas remains limited. Consequently, affecting BTB permeability pharmaceutically may serve as a potential treatment approach. Interestingly, a recent study reported that HOTAIR knockdown resulted in the increase of BTB permeability by targeting miR-148b-3p in glioma microvascular endothelial cells [50]. miR-148b-3p was shown to downregulate the expression of tight junction-related proteins including ZO-1, clauidin-5, and occludin through targeting of USF1 [50]. Meanwhile, miR-148b-3p was demonstrated to target HOTAIR in this study [50]. Hence, the HOTAIR/miR-148b-3p/USF1 pathway may play a pivotal role in drug delivery affecting the permeability of BTB in gliomas and deserves further study [50].
Conclusions
Herein, we explored the role of HOTAIR in glioma development and progression, as well as analyzed the existing evidence regarding the underlying molecular mechanisms. Emerging preclinical and clinical evidence reveals that HOTAIR is overexpressed in human gliomas and mechanistic studies support its pro-oncogenic role, since it has been shown to promote cell proliferation, cell cycle progression/checkpoint bypass, survival, metabolism, invasiveness, and angiogenesis both in vitro and in vivo. Potential underlying mechanisms involve the regulation of the activity of specific transcription factors, such as MXI1, E2F1, ATF5, and ASCL1; the modulation of the expression of cell cycle–associated genes and related pathways, including the Wnt/β-catenin axis; the epigenetic inhibition of PDCD4 gene expression; and the interaction of HOTAIR with specific miRNAs, such as miR-326, miR-141, miR-148b-3p, miR-15b, and miR-126-5p (Figs. 1, 2, and 3). HOTAIR has been also implicated in the TGF-β pathway (through RUNX1) and PI3K/AKT/mTOR pathway (through FGF1 and miR125-a), as well as the MAPK/MEK/ERK pathway (through FGF1). Regarding its clinical significance, high HOTAIR levels in glioma tissues and in the serum of glioma patients have been negatively correlated with overall survival and response to temozolamide, suggesting its potential use as a prognostic biomarker. Finally, targeting HOTAIR via si-HOTAIR delivery into glioma cells or small molecule inhibitors may represent a novel approach toward glioma treatment, paving the way for future research.
However, several limiting aspects need to be further elucidated. Firstly, given the large molecular intratumor heterogeneity of gliomas, the specific role of HOTAIR in forming a tumor microenvironment should be explored [35, 182]. Secondly, it has been shown that most lncRNAs in GBM regulate multiple targets and act in a multimodal manner, being able to enhance or attenuate gene expression or signaling pathways in a target-specific manner [46, 183, 184]. Given the complicated network of molecular interactions between HOTAIR and DNA, RNAs, and proteins, as well as the various loops in which HOTAIR is implicated, its effects should be carefully investigated before the translation of HOTAIR-directed molecules to the clinical setting.
Abbreviations
- HOTAIR:
-
HOX transcript antisense intergenic RNA
- ASCL1:
-
Achaete-scute homolog 1
- ATF5:
-
Activating transcription factor 5
- APC:
-
Adenomatous polyposis coli
- ANRIL:
-
Antisense ncRNA in the INK4 locus
- Bcl-xL:
-
B cell lymphoma-extra-large
- BET:
-
Bromodomain and extraterminal
- BRD4:
-
Bromodomain containing 4
- SNORD76:
-
C/D box snoRNA U76
- CCLE:
-
Cancer Cell Line Encyclopedia
- CNS:
-
Central nervous system
- CGGA:
-
Chinese Glioma Genome Atlas
- CD300A:
-
Cluster of differentiation 300A
- CRNDE:
-
Colorectal neoplasia differentially expressed
- CDK2:
-
Cyclin-dependent kinase 2
- CDK4:
-
Cyclin-dependent kinase 4
- H3K4me2:
-
Dimethylation of histone 3 lysine 4
- ADAM22:
-
Disintegrin and metalloproteinase domain-containing protein 22
- EED:
-
Embryonic ectoderm development
- EZH2:
-
Enhancer of zeste homolog 2
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial–mesenchymal transition
- FGF1:
-
Fibroblast growth factor 1
- FGFR:
-
Fibroblast growth factor receptor
- GSEA:
-
Gene set enrichment analysis
- GWAS:
-
Genome-wide association studies
- GSCs:
-
Glioma stem cells
- GSH:
-
Glutathione
- GSK3β:
-
Glycogen synthase kinase-3β
- GAS5:
-
Growth arrest-specific 5
- HDAC1:
-
Histone deacetylase 1
- HIF-1:
-
Hypoxia-inducible factor-1
- IGF1R:
-
Insulin-like growth factor receptor-1
- IDH:
-
Isocitrate dehydrogenase
- KRAS:
-
Kirsten rat sarcoma viral oncogene
- STAT3:
-
Signal transducer and activator of transcription 3
- lncRNAs:
-
Long ncRNAs
- LRP6:
-
Low-density lipoprotein receptor-related protein 6
- LSD1:
-
Lysine-specific demethylase 1
- mTOR:
-
Mammalian target of rapamycin
- MEG3:
-
Maternally expressed gene 3
- MXI1:
-
MAX-interacting protein 1
- MMPs:
-
Metalloproteinases
- miRNAs:
-
MicroRNAs
- MEK:
-
Mitogen-activated protein kinase kinase
- NLK:
-
Nemo-like kinase
- NCAM1:
-
Neural cell adhesion molecule 1
- TNM:
-
Node and metastasis
- ncRNAs:
-
Noncoding RNAs
- PTCSC3:
-
Papillary thyroid carcinoma susceptibility candidate 3
- PI3K:
-
Phosphoinositide 3-kinase
- piRNAs:
-
Piwi-interacting RNAs
- PRC2:
-
Polycomb-repressive complex 2
- PDCD4:
-
Programmed cell death protein 4
- AKT:
-
Protein kinase B
- PKC:
-
Protein kinase C
- PRKCE:
-
Protein kinase C epsilon type
- qRT-PCR:
-
Quantitative real-time PCR
- REST:
-
RE1-silencing transcription factor
- CoREST1:
-
REST corepressor 1
- RUNX:
-
Runt-related transcription factor 1
- SNPs:
-
Single nucleotide polymorphisms
- siRNAs:
-
Small interfering RNAs
- snRNAs:
-
Small nuclear RNAs
- snoRNAs:
-
Small nucleolar RNAs
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- SUZ12:
-
Suppressor of zeste homolog 12
- TUG1:
-
Taurine upregulated gene 1
- TCGA:
-
The Cancer Genome Atlas
- H3K27me3:
-
Trimethylation of lysine 27 on histone H3
- USF1:
-
Upstream stimulating factor 1
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
References
Bush NA, Chang SM, Berger MS (2017) Current and future strategies for treatment of glioma. Neurosurg Rev 40:1–14
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Angelopoulou E, Piperi C (2018) Emerging role of plexins signaling in glioma progression and therapy. Cancer Lett 414:81–87
Wesseling P, Capper D (2018) WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol 44:139–150
Masui K, Mischel PS, Reifenberger G (2016) Molecular classification of gliomas. Handb Clin Neurol 134:97–120
Angelopoulou E, Paudel YN, Piperi C (2019) Emerging pathogenic and prognostic significance of paired box 3 (PAX3) protein in adult gliomas. Transl Oncol 12:1357–1363
Consortium EP (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74
Gomes AQ, Nolasco S, Soares H (2013) Non-coding RNAs: multi-tasking molecules in the cell. Int J Mol Sci 14:16010–16039
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10:155–159
Liu SJ, Nowakowski TJ, Pollen AA, Lui JH, Horlbeck MA, Attenello FJ, He D, Weissman JS, Kriegstein AR, Diaz AA et al (2016) Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome Biol 17:67
Geisler S, Coller J (2013) RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14:699–712
Moran VA, Perera RJ, Khalil AM (2012) Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Res 40:6391–6400
Dong X, Jin Z, Chen Y, Xu H, Ma C, Hong X, Li Y, Zhao G (2018) Knockdown of long non-coding RNA ANRIL inhibits proliferation, migration, and invasion but promotes apoptosis of human glioma cells by upregulation of miR-34a. J Cell Biochem 119:2708–2718
Zhen L, Yun-Hui L, Hong-Yu D, Jun M, Yi-Long Y (2016) Long noncoding RNA NEAT1 promotes glioma pathogenesis by regulating miR-449b-5p/c-met axis. Tumour Biol 37:673–683
Wang P, Ren Z, Sun P (2012) Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem 113:1868–1874
Qin N, Tong GF, Sun LW, Xu XL (2017) Long noncoding RNA MEG3 suppresses glioma cell proliferation, migration, and invasion by acting as a competing endogenous RNA of miR-19a. Oncol Res 25:1471–1478
Gong X, Huang MY (2020) Tumor-suppressive function of lncRNA-MEG3 in glioma cells by regulating miR-6088/SMARCB1 axis. Biomed Res Int 2020:4309161
Shang C, Guo Y, Hong Y, Xue YX (2016) Long non-coding RNA TUSC7, a target of miR-23b, plays tumor-suppressing roles in human gliomas. Front Cell Neurosci 10:235
Bhan A, Mandal SS (2015) LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta 1856:151–164
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL et al (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464:1071–1076
Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A et al (2009) Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 106:11667–11672
Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329:689–693
Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C, Ayad NG (2018) Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer 17:74
Mozdarani H, Ezzatizadeh V, Rahbar Parvaneh R (2020) The emerging role of the long non-coding RNA HOTAIR in breast cancer development and treatment. J Transl Med 18:152
Carrion K, Dyo J, Patel V, Sasik R, Mohamed SA, Hardiman G, Nigam V (2014) The long non-coding HOTAIR is modulated by cyclic stretch and WNT/beta-CATENIN in human aortic valve cells and is a novel repressor of calcification genes. PLoS One 9:e96577
Yoon JH, Abdelmohsen K, Kim J, Yang X, Martindale JL, Tominaga-Yamanaka K, White EJ, Orjalo AV, Rinn JL, Kreft SG et al (2013) Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat Commun 4:2939
Xia M, Yao L, Zhang Q, Wang F, Mei H, Guo X, Huang W (2017) Long noncoding RNA HOTAIR promotes metastasis of renal cell carcinoma by up-regulating histone H3K27 demethylase JMJD3. Oncotarget 8:19795–19802
Zhong DN, Luo YH, Mo WJ, Zhang X, Tan Z, Zhao N, Pang SM, Chen G, Rong MH, Tang W (2018) High expression of long noncoding HOTAIR correlated with hepatocarcinogenesis and metastasis. Mol Med Rep 17:1148–1156
Lu R, Zhang J, Zhang W, Huang Y, Wang N, Zhang Q, Qu S (2018) Circulating HOTAIR expression predicts the clinical response to neoadjuvant chemotherapy in patients with breast cancer. Cancer Biomark 22:249–256
Yan TH, Lu SW, Huang YQ, Que GB, Chen JH, Chen YP, Zhang HB, Liang XL, Jiang JH (2014) Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage ta/T1 bladder cancer. Tumour Biol 35:10249–10257
Balci T, Yilmaz Susluer S, Kayabasi C, Ozmen Yelken B, Biray Avci C, Gunduz C (2016) Analysis of dysregulated long non-coding RNA expressions in glioblastoma cells. Gene 590:120–122
Rynkeviciene R, Simiene J, Strainiene E, Stankevicius V, Usinskiene J, Miseikyte Kaubriene E, Meskinyte I, Cicenas J, Suziedelis K (2018) Non-coding RNAs in glioma. Cancers (Basel) 11. https://doi.org/10.3390/cancers11010017
Li J, Zhu Y, Wang H, Ji X (2018) Targeting long noncoding RNA in glioma: a pathway perspective. Mol Therapy Nucleic Acids 13:431–441
Malissovas N, Ninou E, Michail A, Politis PK (2019) Targeting long non-coding RNAs in nervous system cancers: new insights in prognosis, diagnosis and therapy. Curr Med Chem 26:5649–5663
Shi J, Dong B, Cao J, Mao Y, Guan W, Peng Y, Wang S (2017) Long non-coding RNA in glioma: signaling pathways. Oncotarget 8:27582–27592
Liang H, Huang W, Wang Y, Ding L, Zeng L (2019) Overexpression of MiR-146a-5p upregulates lncRNA HOTAIR in triple-negative breast cancer cells and predicts poor prognosis. Technol Cancer Res Treatment 18:1533033819882949
Dong X, He X, Guan A, Huang W, Jia H, Huang Y, Chen S, Zhang Z, Gao J, Wang H (2019) Long non-coding RNA Hotair promotes gastric cancer progression via miR-217-GPC5 axis. Life Sci 217:271–282
Wu Y, Liu J, Zheng Y, You L, Kuang D, Liu T (2014) Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol 35:11887–11894
Ke J, Yao YL, Zheng J, Wang P, Liu YH, Ma J, Li Z, Liu XB, Li ZQ, Wang ZH et al (2015) Knockdown of long non-coding RNA HOTAIR inhibits malignant biological behaviors of human glioma cells via modulation of miR-326. Oncotarget 6:21934–21949
Pastori C, Kapranov P, Penas C, Peschansky V, Volmar CH, Sarkaria JN, Bregy A, Komotar R, St Laurent G, Ayad NG et al (2015) The Bromodomain protein BRD4 controls HOTAIR, a long noncoding RNA essential for glioblastoma proliferation. Proc Natl Acad Sci U S A 112:8326–8331
Zhang K, Sun X, Zhou X, Han L, Chen L, Shi Z, Zhang A, Ye M, Wang Q, Liu C et al (2015) Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6:537–546
Zhou X, Ren Y, Zhang J, Zhang C, Zhang K, Han L, Kong L, Wei J, Chen L, Yang J et al (2015) HOTAIR is a therapeutic target in glioblastoma. Oncotarget 6:8353–8365
Chen L, Han L, Wei J, Zhang K, Shi Z, Duan R, Li S, Zhou X, Pu P, Zhang J et al (2015) SNORD76, a box C/D snoRNA, acts as a tumor suppressor in glioblastoma. Sci Rep 5:8588
Chen Y, Bian Y, Zhao S, Kong F, Li X (2016) Suppression of PDCD4 mediated by the long non-coding RNA HOTAIR inhibits the proliferation and invasion of glioma cells. Oncol Lett 12:5170–5176
Wang G, Li Z, Tian N, Han L, Fu Y, Guo Z, Tian Y (2016) miR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol Lett 12:879–886
Li Y, Wang Z, Wang Y, Zhao Z, Zhang J, Lu J, Xu J, Li X (2016) Identification and characterization of lncRNA mediated transcriptional dysregulation dictates lncRNA roles in glioblastoma. Oncotarget 7:45027–45041
Fang K, Liu P, Dong S, Guo Y, Cui X, Zhu X, Li X, Jiang L, Liu T, Wu Y (2016) Magnetofection based on superparamagnetic iron oxide nanoparticle-mediated low lncRNA HOTAIR expression decreases the proliferation and invasion of glioma stem cells. Int J Oncol 49:509–518
Bian EB, Ma CC, He XJ, Wang C, Zong G, Wang HL, Zhao B (2016) Epigenetic modification of miR-141 regulates SKA2 by an endogenous ‘sponge’ HOTAIR in glioma. Oncotarget 7:30610–30625
Huang K, Sun J, Yang C, Wang Y, Zhou B, Kang C, Han L, Wang Q (2017) HOTAIR upregulates an 18-gene cell cycle-related mRNA network in glioma. Int J Oncol. https://doi.org/10.3892/ijo.2017.3901
Sa L, Li Y, Zhao L, Liu Y, Wang P, Liu L, Li Z, Ma J, Cai H, Xue Y (2017) The role of HOTAIR/miR-148b-3p/USF1 on regulating the permeability of BTB. Front Mol Neurosci 10:194
Jiang Y, Zhang Q, Bao J, Du C, Wang J, Tong Q, Liu C (2017) Schisandrin B inhibits the proliferation and invasion of glioma cells by regulating the HOTAIR-micoRNA-125a-mTOR pathway. Neuroreport 28:93–100
Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD, Qin YY, Gong W, Quan ZW (2014) Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer 13:156
Xavier-Magalhaes A, Oliveira AI, de Castro JV, Pojo M, Goncalves CS, Lourenco T, Viana-Pereira M, Costa S, Linhares P, Vaz R et al (2017) Effects of the functional HOTAIR rs920778 and rs12826786 genetic variants in glioma susceptibility and patient prognosis. J Neuro-Oncol 132:27–34
Xavier-Magalhaes A, Goncalves CS, Fogli A, Lourenco T, Pojo M, Pereira B, Rocha M, Lopes MC, Crespo I, Rebelo O et al (2018) The long non-coding RNA HOTAIR is transcriptionally activated by HOXA9 and is an independent prognostic marker in patients with malignant glioma. Oncotarget 9:15740–15756
Sun G, Wang Y, Zhang J, Lin N, You Y (2018) MiR-15b/HOTAIR/p53 form a regulatory loop that affects the growth of glioma cells. J Cell Biochem 119:4540–4547
Liu L, Cui S, Wan T, Li X, Tian W, Zhang R, Luo L, Shi Y (2018) Long non-coding RNA HOTAIR acts as a competing endogenous RNA to promote glioma progression by sponging miR-126-5p. J Cell Physiol 233:6822–6831
Liu Y, Jiang H, Zhou H, Ying X, Wang Z, Yang Y, Xu W, He X, Li Y (2018) Lentivirus-mediated silencing of HOTAIR lncRNA restores gefitinib sensitivity by activating Bax/Caspase-3 and suppressing TGF-alpha/EGFR signaling in lung adenocarcinoma. Oncol Lett 15:2829–2838
Yang B, Wei ZY, Wang BQ, Yang HC, Wang JY, Bu XY (2018) Down-regulation of the long noncoding RNA-HOX transcript antisense intergenic RNA inhibits the occurrence and progression of glioma. J Cell Biochem 119:2278–2287
Lei B, Yu L, Jung TA, Deng Y, Xiang W, Liu Y, Qi S (2018) Prospective series of nine long noncoding RNAs associated with survival of patients with glioblastoma. J Neurol Surg Part A 79:471–478
Shen J, Hodges TR, Song R, Gong Y, Calin GA, Heimberger AB, Zhao H (2018) Serum HOTAIR and GAS5 levels as predictors of survival in patients with glioblastoma. Mol Carcinog 57:137–141
Li Z, Tan H, Zhao W, Xu Y, Zhang Z, Wang M, Zhou X (2019a) Integrative analysis of DNA methylation and gene expression profiles identifies MIR4435-2HG as an oncogenic lncRNA for glioma progression. Gene 715:144012
Li Y, Ren Y, Wang Y, Tan Y, Wang Q, Cai J, Zhou J, Yang C, Zhao K, Yi K et al (2019b) A compound AC1Q3QWB selectively disrupts HOTAIR-mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics 9:4608–4623
Zhao WH, Yuan HY, Ren XY, Huang K, Guo ZY (2019a) Association between expression of HOTAIR and invasiveness of gliomas, and its predictive value. Adv Clin Exp Med 28:1179–1183
Zhao K, Cui X, Wang Q, Fang C, Tan Y, Wang Y, Yi K, Yang C, You H, Shang R et al (2019b) RUNX1 contributes to the mesenchymal subtype of glioblastoma in a TGFbeta pathway-dependent manner. Cell Death Dis 10:877
Shi J, Lv S, Wu M, Wang X, Deng Y, Li Y, Li K, Zhao H, Zhu X, Ye M (2020) HOTAIR-EZH2 inhibitor AC1Q3QWB upregulates CWF19L1 and enhances cell cycle inhibition of CDK4/6 inhibitor palbociclib in glioma. Clin Translat Med 10:182–198
Zhang L, He A, Chen B, Bi J, Chen J, Guo D, Qian Y, Wang W, Shi T, Zhao Z et al (2020) A HOTAIR regulatory element modulates glioma cell sensitivity to temozolomide through long-range regulation of multiple target genes. Genome Res. https://doi.org/10.1101/gr.251058.119
Zhang J, Chen G, Gao Y, Liang H (2020) HOTAIR/miR-125 axis-mediated hexokinase 2 expression promotes chemoresistance in human glioblastoma. J Cell Mol Med 24:5707–5717
Yuan Z, Yang Z, Li W, Wu A, Su Z, Jiang B (2020) Exosome-mediated transfer of long noncoding RNA HOTAIR regulates temozolomide resistance by miR-519a-3p/RRM1 axis in glioblastoma. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2019.3499
Li H, Guan C (2020) HOTAIR inhibits the proliferation of glioblastoma cells by targeting miR-219. Cancer Biomark 28:41–47
Lu L, Zhu G, Zhang C, Deng Q, Katsaros D, Mayne ST, Risch HA, Mu L, Canuto EM, Gregori G et al (2012) Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat 136:875–883
Hajjari M, Salavaty A (2015) HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med 12:1–9
Bhan A, Hussain I, Ansari KI, Bobzean SA, Perrotti LI, Mandal SS (2014) Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol Biol 141:160–170
Padua Alves C, Fonseca AS, Muys BR, de Barros ELBR, Burger MC, de Souza JE, Valente V, Zago MA, Silva WA Jr (2013) Brief report: the lincRNA Hotair is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells 31:2827–2832
Zhuang Y, Wang X, Nguyen HT, Zhuo Y, Cui X, Fewell C, Flemington EK, Shan B (2013) Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen. J Hematol Oncol 6:35
Cancer Genome Atlas Research N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Pojo M, Goncalves CS, Xavier-Magalhaes A, Oliveira AI, Goncalves T, Correia S, Rodrigues AJ, Costa S, Pinto L, Pinto AA et al (2015) A transcriptomic signature mediated by HOXA9 promotes human glioblastoma initiation, aggressiveness and resistance to temozolomide. Oncotarget 6:7657–7674
Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN (2008) c-Myc is required for maintenance of glioma cancer stem cells. PLoS One 3:e3769
Kaminska B, Kocyk M, Kijewska M (2013) TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol 986:171–187
Zhang JX, Han L, Bao ZS, Wang YY, Chen LY, Yan W, Yu SZ, Pu PY, Liu N, You YP et al (2013) HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro-Oncology 15:1595–1603
Wenzel ES, Singh ATK (2018) Cell-cycle checkpoints and aneuploidy on the path to cancer. In Vivo 32:1–5
Fischer M, Quaas M, Steiner L, Engeland K (2016) The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle genes. Nucleic Acids Res 44:164–174
Ohta S, Kimura M, Takagi S, Toramoto I, Ishihama Y (2016) Identification of mitosis-specific phosphorylation in mitotic chromosome-associated proteins. J Proteome Res 15:3331–3341
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP et al (2002) The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419:624–629
Orzan F, Pellegatta S, Poliani PL, Pisati F, Caldera V, Menghi F, Kapetis D, Marras C, Schiffer D, Finocchiaro G (2011) Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol Appl Neurobiol 37:381–394
Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L et al (2009) EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res 69:9211–9218
Zhang R, Wang R, Chang H, Wu F, Liu C, Deng D, Fan W (2012) Downregulation of Ezh2 expression by RNA interference induces cell cycle arrest in the G0/G1 phase and apoptosis in U87 human glioma cells. Oncol Rep 28:2278–2284
Kim E, Kim M, Woo DH, Shin Y, Shin J, Chang N, Oh YT, Kim H, Rheey J, Nakano I et al (2013) Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 23:839–852
Liu H, Sun Y, Qi X, Gordon RE, O'Brien JA, Yuan H, Zhang J, Wang Z, Zhang M, Song Y et al (2019) EZH2 phosphorylation promotes self-renewal of glioma stem-like cells through NF-kappaB methylation. Front Oncol 9:641
Zhang K, Zhang J, Han L, Pu P, Kang C (2012) Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol 7:740–749
Liu HW, Su YK, Bamodu OA, Hueng DY, Lee WH, Huang CC, Deng L, Hsiao M, Chien MH, Yeh CT et al (2018) The disruption of the beta-catenin/TCF-1/STAT3 signaling axis by 4-acetylantroquinonol B inhibits the tumorigenesis and cancer stem-cell-like properties of glioblastoma cells, in vitro and in vivo cancers. Cancers (Basel) 10. https://doi.org/10.3390/cancers10120491
Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H et al (1999) The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399:798–802
Gan HK, Kaye AH, Luwor RB (2009) The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci 16:748–754
Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K (2005) Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 11:1462–1466
Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim J, Cha HJ (2013) Loss of E-cadherin activates EGFR-MEK/ERK signaling, which promotes invasion via the ZEB1/MMP2 axis in non-small cell lung cancer. Oncotarget 4:2512–2522
Wang Y, Lin Z, Sun L, Fan S, Huang Z, Zhang D, Yang Z, Li J, Chen W (2014) Akt/Ezrin Tyr353/NF-kappaB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer 110:695–705
Vesuna F, van Diest P, Chen JH, Raman V (2008) Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun 367:235–241
Jia L, Tian Y, Chen Y, Zhang G (2018) The silencing of LncRNA-H19 decreases chemoresistance of human glioma cells to temozolomide by suppressing epithelial-mesenchymal transition via the Wnt/beta-catenin pathway. OncoTargets Therapy 11:313–321
Xia S, Ji R, Zhan W (2017) Long noncoding RNA papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) inhibits proliferation and invasion of glioma cells by suppressing the Wnt/beta-catenin signaling pathway. BMC Neurol 17:30
Xiao D, Cui X, Wang X (2019) LncRNA PTCSC3 inhibits cell proliferation in laryngeal squamous cell carcinoma by down-regulating lncRNA HOTAIR. Biosci Rep 39. https://doi.org/10.1042/BSR20182362
Liwak U, Jordan LE, Von-Holt SD, Singh P, Hanson JE, Lorimer IA, Roncaroli F, Holcik M (2013) Loss of PDCD4 contributes to enhanced chemoresistance in glioblastoma multiforme through de-repression of Bcl-xL translation. Oncotarget 4:1365–1372
Gaur AB, Holbeck SL, Colburn NH, Israel MA (2011) Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro-oncology 13:580–590
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Yang L, Lin C, Liu W, Zhang J, Ohgi KA, Grinstein JD, Dorrestein PC, Rosenfeld MG (2011) ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 147:773–788
Manni I, Tunici P, Cirenei N, Albarosa R, Colombo BM, Roz L, Sacchi A, Piaggio G, Finocchiaro G (2002) Mxi1 inhibits the proliferation of U87 glioma cells through down-regulation of cyclin B1 gene expression. Br J Cancer 86:477–484
Xu S, Wen Z, Jiang Q, Zhu L, Feng S, Zhao Y, Wu J, Dong Q, Mao J, Zhu Y (2015) CD58, a novel surface marker, promotes self-renewal of tumor-initiating cells in colorectal cancer. Oncogene 34:1520–1531
Sharif TR, Sharif M (1999) Overexpression of protein kinase C epsilon in astroglial brain tumor derived cell lines and primary tumor samples. Int J Oncol 15:237–243
Safaee M, Clark AJ, Oh MC, Ivan ME, Bloch O, Kaur G, Sun MZ, Kim JM, Oh T, Berger MS et al (2013) Overexpression of CD97 confers an invasive phenotype in glioblastoma cells and is associated with decreased survival of glioblastoma patients. PLoS One 8:e62765
Angelastro JM, Canoll PD, Kuo J, Weicker M, Costa A, Bruce JN, Greene LA (2006) Selective destruction of glioblastoma cells by interference with the activity or expression of ATF5. Oncogene 25:907–916
Wang Z, Dai X, Chen Y, Sun C, Zhu Q, Zhao H, Liu G, Huang Q, Lan Q (2015) MiR-30a-5p is induced by Wnt/beta-catenin pathway and promotes glioma cell invasion by repressing NCAM. Biochem Biophys Res Commun 465:374–380
D'Abaco GM, Ng K, Paradiso L, Godde NJ, Kaye A, Novak U (2006) ADAM22, expressed in normal brain but not in high-grade gliomas, inhibits cellular proliferation via the disintegrin domain. Neurosurgery 58:179–186; discussion 179-186
Park NI, Guilhamon P, Desai K, McAdam RF, Langille E, O'Connor M, Lan X, Whetstone H, Coutinho FJ, Vanner RJ et al (2017) ASCL1 reorganizes chromatin to direct neuronal fate and suppress tumorigenicity of glioblastoma stem cells. Cell Stem Cell 21(209–224):e207
Xiao D, Huang J, Pan Y, Li H, Fu C, Mao C, Cheng Y, Shi Y, Chen L, Jiang Y et al (2017) Chromatin remodeling factor LSH is upregulated by the LRP6-GSK3beta-E2F1 axis linking reversely with survival in gliomas. Theranostics 7:132–143
Suzuki N, Idogawa M, Tange S, Ohashi T, Sasaki Y, Nakase H, Tokino T (2020) p53-induced ARVCF modulates the splicing landscape and supports the tumor suppressive function of p53. Oncogene 39:2202–2211
Zhi T, Jiang K, Xu X, Yu T, Zhou F, Wang Y, Liu N, Zhang J (2019) ECT2/PSMD14/PTTG1 axis promotes the proliferation of glioma through stabilizing E2F1. Neuro-oncology 21:462–473
Held-Feindt J, Hattermann K, Knerlich-Lukoschus F, Mehdorn HM, Mentlein R (2011) SP100 reduces malignancy of human glioma cells. Int J Oncol 38:1023–1030
Bogoch Y, Friedlander-Malik G, Lupu L, Bondar E, Zohar N, Langier S, Ram Z, Nachmany I, Klausner JM, Pencovich N (2017) Augmented expression of RUNX1 deregulates the global gene expression of U87 glioblastoma multiforme cells and inhibits tumor growth in mice. Tumour Biol 39:1010428317698357
Yoon JH, Abdelmohsen K, Gorospe M (2014) Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol 34:9–14
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH et al (2014) Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer 13:92
Zhang H, Cai K, Wang J, Wang X, Cheng K, Shi F, Jiang L, Zhang Y, Dou J (2014) MiR-7, inhibited indirectly by lincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells 32:2858–2868
Kefas B, Comeau L, Erdle N, Montgomery E, Amos S, Purow B (2010) Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro-oncology 12:1102–1112
Hsu YC, Kao CY, Chung YF, Lee DC, Liu JW, Chiu IM (2016) Activation of Aurora A kinase through the FGF1/FGFR signaling axis sustains the stem cell characteristics of glioblastoma cells. Exp Cell Res 344:153–166
Xin Z, Song X, Jiang B, Gongsun X, Song L, Qin Q, Wang Q, Shi M, Liu X (2018) Blocking FGFR4 exerts distinct anti-tumorigenic effects in esophageal squamous cell carcinoma. Thoracic Cancer 9:1687–1698
Packer LM, Geng X, Bonazzi VF, Ju RJ, Mahon CE, Cummings MC, Stephenson SA, Pollock PM (2017) PI3K inhibitors synergize with FGFR inhibitors to enhance antitumor responses in FGFR2(mutant) endometrial cancers. Mol Cancer Ther 16:637–648
Zanotto-Filho A, Goncalves RM, Klafke K, de Souza PO, Dillenburg FC, Carro L, Gelain DP, Moreira JC (2017) Inflammatory landscape of human brain tumors reveals an NFkappaB dependent cytokine pathway associated with mesenchymal glioblastoma. Cancer Lett 390:176–187
Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y, Enokida H et al (2013) Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One 8:e70372
Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M et al (2014) Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem 289:12550–12565
Hanisch A, Sillje HH, Nigg EA (2006) Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J 25:5504–5515
He XJ, Bian EB, Ma CC, Wang C, Wang HL, Zhao B (2018) Long non-coding RNA SPRY4-IT1 promotes the proliferation and invasion of U251 cells through upregulation of SKA2. Oncol Lett 15:3977–3984
Zhao G, Zhang JG, Liu Y, Qin Q, Wang B, Tian K, Liu L, Li X, Niu Y, Deng SC et al (2013) miR-148b functions as a tumor suppressor in pancreatic cancer by targeting AMPKalpha1. Mol Cancer Ther 12:83–93
Chang H, Zhou X, Wang ZN, Song YX, Zhao F, Gao P, Chiang Y, Xu HM (2012) Increased expression of miR-148b in ovarian carcinoma and its clinical significance. Mol Med Rep 5:1277–1280
Wang J, Liu H, Tian L, Wang F, Han L, Zhang W, Bai YA (2017) miR-15b inhibits the progression of glioblastoma cells through targeting insulin-like growth factor receptor 1. Hormones Cancer 8:49–57
Zhang Y, Dube C, Gibert M Jr, Cruickshanks N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K et al (2018) The p53 pathway in glioblastoma. Cancers 10. https://doi.org/10.3390/cancers10090297
Szeliga M, Albrecht J (2015) Opposing roles of glutaminase isoforms in determining glioblastoma cell phenotype. Neurochem Int 88:6–9
Petovari G, Danko T, Krencz I, Hujber Z, Rajnai H, Vetlenyi E, Raffay R, Papay J, Jeney A, Sebestyen A (2019) Inhibition of metabolic shift can decrease therapy resistance in human high-grade glioma cells. Pathol Oncol Res. https://doi.org/10.1007/s12253-019-00677-2
Luan Y, Zuo L, Zhang S, Wang G, Peng T (2015) MicroRNA-126 acts as a tumor suppressor in glioma cells by targeting insulin receptor substrate 1 (IRS-1). Int J Clin Exp Pathol 8:10345–10354
Li Y, Li Y, Ge P, Ma C (2017) MiR-126 regulates the ERK pathway via targeting KRAS to inhibit the glioma cell proliferation and invasion. Mol Neurobiol 54:137–145
Jiang Y, Zhang Q, Bao J, Du C, Wang J, Tong Q, Liu C (2015) Schisandrin B suppresses glioma cell metastasis mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed Pharmacotherapy 74:77–82
Yuan J, Xiao G, Peng G, Liu D, Wang Z, Liao Y, Liu Q, Wu M, Yuan X (2015) MiRNA-125a-5p inhibits glioblastoma cell proliferation and promotes cell differentiation by targeting TAZ. Biochem Biophys Res Commun 457:171–176
Tang L, Shen H, Li X, Li Z, Liu Z, Xu J, Ma S, Zhao X, Bai X, Li M et al (2016) MiR-125a-5p decreases after long non-coding RNA HOTAIR knockdown to promote cancer cell apoptosis by releasing caspase 2. Cell Death Dis 7:e2137
Rao SA, Arimappamagan A, Pandey P, Santosh V, Hegde AS, Chandramouli BA, Somasundaram K (2013) miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PLoS One 8:e63164
Yoshihama M, Nakao A, Kenmochi N (2013) snOPY: a small nucleolar RNA orthological gene database. BMC Res Notes 6:426
Guarnaccia L, Navone SE, Trombetta E, Cordiglieri C, Cherubini A, Crisa FM, Rampini P, Miozzo M, Fontana L, Caroli M et al (2018) Angiogenesis in human brain tumors: screening of drug response through a patient-specific cell platform for personalized therapy. Sci Rep 8:8748
Plate KH, Breier G, Weich HA, Mennel HD, Risau W (1994) Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 59:520–529
Subramaniam SR, Federoff HJ (2017) Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci 9:176
Fu WM, Lu YF, Hu BG, Liang WC, Zhu X, Yang HD, Li G, Zhang JF (2016) Long noncoding RNA Hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways. Oncotarget 7:4712–4723
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-oncology 7:134–153
Hong Q, Li O, Zheng W, Xiao WZ, Zhang L, Wu D, Cai GY, He JC, Chen XM (2017) LncRNA HOTAIR regulates HIF-1alpha/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis 8:e2772
Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N (2014) Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int 38:1076–1079
Wang S, Zhang X, Guo Y, Rong H, Liu T (2017) The long noncoding RNA HOTAIR promotes Parkinson's disease by upregulating LRRK2 expression. Oncotarget 8:24449–24456
Pahlevan Kakhki M, Nikravesh A, Shirvani Farsani Z, Sahraian MA, Behmanesh M (2018) HOTAIR but not ANRIL long non-coding RNA contributes to the pathogenesis of multiple sclerosis. Immunology 153:479–487
Wang W, He X, Zheng Z, Ma X, Hu X, Wu D, Wang M (2017) Serum HOTAIR as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Mol Cancer 16:75
Gao JZ, Li J, Du JL, Li XL (2016) Long non-coding RNA HOTAIR is a marker for hepatocellular carcinoma progression and tumor recurrence. Oncol Lett 11:1791–1798
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W (2013) The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 13:464
Sorensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, Larsen MJ, Kruse TA (2013) Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast Cancer Res Treat 142:529–536
Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z, Protivankova M et al (2014) HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 35:1510–1515
Xu Z, Chen H, Yang B, Liu X, Zhou X, Kong H (2019) The association of HOTAIR with the diagnosis and prognosis of gastric cancer and its effect on the proliferation of gastric cancer cells. Can J Gastroenterol Hepatol 2019:3076345
Toy HI, Okmen D, Kontou PI, Georgakilas AG, Pavlopoulou A (2019) HOTAIR as a prognostic predictor for diverse human cancers: a meta- and bioinformatics analysis. Cancers (Basel) 11. https://doi.org/10.3390/cancers11060778
Zhang JX, Zhang J, Yan W, Wang YY, Han L, Yue X, Liu N, You YP, Jiang T, Pu PY et al (2013) Unique genome-wide map of TCF4 and STAT3 targets using ChIP-seq reveals their association with new molecular subtypes of glioblastoma. Neuro-oncology 15:279–289
Zhang C, Moore LM, Li X, Yung WK, Zhang W (2013) IDH1/2 mutations target a key hallmark of cancer by deregulating cellular metabolism in glioma. Neuro-oncology 15:1114–1126
Li J, Wang Y, Yu J, Dong R, Qiu H (2015) A high level of circulating HOTAIR is associated with progression and poor prognosis of cervical cancer. Tumour Biol 36:1661–1665
Basu B, Ghosh MK (2019) Extracellular vesicles in glioma: from diagnosis to therapy. BioEssays 41:e1800245
Ghasimi S, Wibom C, Dahlin AM, Brannstrom T, Golovleva I, Andersson U, Melin B (2016) Genetic risk variants in the CDKN2A/B, RTEL1 and EGFR genes are associated with somatic biomarkers in glioma. J Neuro-Oncol 127:483–492
Vieira de Castro J, Goncalves CS, Costa S, Linhares P, Vaz R, Nabico R, Amorim J, Viana-Pereira M, Reis RM, Costa BM (2015) Impact of TGF-beta1 -509C/T and 869T/C polymorphisms on glioma risk and patient prognosis. Tumour Biol 36:6525–6532
Bayram S, Sumbul AT, Batmaci CY, Genc A (2015) Effect of HOTAIR rs920778 polymorphism on breast cancer susceptibility and clinicopathologic features in a Turkish population. Tumour Biol 36:3863–3870
Bayram S, Sumbul AT, Dadas E (2016) A functional HOTAIR rs12826786 C>T polymorphism is associated with breast cancer susceptibility and poor clinicopathological characteristics in a Turkish population: a hospital-based case-control study. Tumour Biol 37:5577–5584
Zhang X, Zhou L, Fu G, Sun F, Shi J, Wei J, Lu C, Zhou C, Yuan Q, Yang M (2014) The identification of an ESCC susceptibility SNP rs920778 that regulates the expression of lncRNA HOTAIR via a novel intronic enhancer. Carcinogenesis 35:2062–2067
Pan W, Liu L, Wei J, Ge Y, Zhang J, Chen H, Zhou L, Yuan Q, Zhou C, Yang M (2016) A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol Carcinog 55:90–96
Guo W, Dong Z, Bai Y, Guo Y, Shen S, Kuang G, Xu J (2015) Associations between polymorphisms of HOTAIR and risk of gastric cardia adenocarcinoma in a population of north China. Tumour Biol 36:2845–2854
Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM, Kumar AP, Challagundla KB, Sethi G, Gupta SC (2019) Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci 76:1947–1966
Chen J, Lin C, Yong W, Ye Y, Huang Z (2015) Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem 35:722–728
Yazdani Y, Sharifi Rad MR, Taghipour M, Chenari N, Ghaderi A, Razmkhah M (2016) Genistein suppression of matrix metalloproteinase 2 (MMP-2) and vascular endothelial growth factor (VEGF) expression in mesenchymal stem cell like cells isolated from high and low grade gliomas. Asian Pac J Cancer Prev 17:5303–5307
Yakisich JS, Ohlsson Lindblom I, Siden A, Cruz MH (2009) Rapid inhibition of ongoing DNA synthesis in human glioma tissue by genistein. Oncol Rep 22:569–574
Imai-Sumida M, Chiyomaru T, Majid S, Saini S, Nip H, Dahiya R, Tanaka Y, Yamamura S (2017) Silibinin suppresses bladder cancer through down-regulation of actin cytoskeleton and PI3K/Akt signaling pathways. Oncotarget 8:92032–92042
Chakrabarti M, Ray SK (2016) Anti-tumor activities of luteolin and silibinin in glioblastoma cells: overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 21:312–328
Kwiatkowska A, Symons M (2020) Signaling determinants of glioma cell invasion. Adv Exp Med Biol 1202:129–149
Chen H, Xin Y, Zhou L, Huang JM, Tao L, Cheng L, Tian J (2014) Cisplatin and paclitaxel target significant long noncoding RNAs in laryngeal squamous cell carcinoma. Med Oncol 31:246
Li CH, Chen Y (2013) Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol 45:1895–1910
Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W (2019) Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res 9:1354–1366
Jin L, Wang Q, Chen J, Wang Z, Xin H, Zhang D (2019) Efficient delivery of therapeutic siRNA by Fe3O4 magnetic nanoparticles into oral cancer cells. Pharmaceutics 11. https://doi.org/10.3390/pharmaceutics11110615
Bruniaux J, Allard-Vannier E, Aubrey N, Lakhrif Z, Ben Djemaa S, Eljack S, Marchais H, Herve-Aubert K, Chourpa I, David S (2019) Magnetic nanocarriers for the specific delivery of siRNA: contribution of breast cancer cells active targeting for down-regulation efficiency. Int J Pharm 569:118572
Sandmann T, Bourgon R, Garcia J, Li C, Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R et al (2015) Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol 33:2735–2744
Strepkos D, Markouli M, Klonou A, Piperi C, Papavassiliou AG (2020 Jan) Insights in the immunobiology of glioblastoma. J Mol Med (Berl) 98(1):1–10
Nikaki A, Piperi C, Papavassiliou AG (2012 Oct) Role of microRNAs in gliomagenesis: targeting miRNAs in glioblastoma multiforme therapy. Expert Opin Investig Drugs 21(10):1475–1488
Paulmurugan R, Malhotra M, Massoud TF (2019 Jul) The protean world of non-coding RNAs in glioblastoma. J Mol Med (Berl) 97(7):909–925
Acknowledgments
YNP would like to acknowledge Monash University Malaysia for supporting with HDR Scholarship.
Author information
Authors and Affiliations
Contributions
EA carried out the literature review and conceptualized and prepared the initial draft. YNP edited and contributed in the final manuscript. CP provided critical inputs and edited and contributed to the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angelopoulou, E., Paudel, Y.N. & Piperi, C. Critical role of HOX transcript antisense intergenic RNA (HOTAIR) in gliomas. J Mol Med 98, 1525–1546 (2020). https://doi.org/10.1007/s00109-020-01984-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01984-x