Abstract
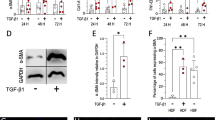
Abnormal scarring is a major challenge in modern medicine. The central role of myofibroblasts and TGF-β signaling in scarring is widely accepted, but effective treatment options are missing. Autologous fat grafting is a novel approach that has led to significant improvements in the functionality and appearance of scar tissue. While the underlying mechanism is unknown, the potential role of paracrine effects of adipocytes has been discussed. Hence, with the aim of unraveling the regenerative potential of adipocytes, their effects on in vitro differentiated myofibroblasts and on fibroblasts from hypertrophic scars were investigated. Exposure to adipocyte-conditioned medium significantly decreased the expression of the myofibroblast marker α-SMA and ECM components, indicating the occurrence of myofibroblast reprogramming. Further analysis demonstrated that myofibroblast reprogramming was triggered by BMP-4 and activation of PPARγ signaling initiating tissue remodeling. These findings may pave the way for novel therapeutic strategies for the prevention or treatment of hypertrophic scars.
Key messages
-
Adipocytes induce distinct regenerative effects in hypertrophic scar tissue.
-
Adipocytes secrete several proteins which are involved in wound healing and regeneration.
-
Adipocytes secrete BMP-4 which activates myofibroblast reprogramming.
-
Mediators secreted by adipocytes directly and indirectly activate PPARγ which exerts distinct anti-fibrotic effects.
-
These findings may pave the way for novel therapeutic strategies for the prevention or treatment of hypertrophic scars.







Similar content being viewed by others
References
Mokos ZB, Jovic A, Grgurevic L, Dumic-Cule I, Kostovic K, Ceovic R, Marinovic B (2017) Current therapeutic approach to hypertrophic scars. Front Med (Lausanne) 4:83
Hinz B (2016) The role of myofibroblasts in wound healing. Curr Res Transl Med 64(4):171–177
Sarrazy V, Billet F, Micallef L, Coulomb B, Desmouliere A (2011) Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen 19(Suppl 1):s10–s15
Hecker L, Jagirdar R, Jin T, Thannickal VJ (2011) Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res 317(13):1914–1921
Garrison G, Huang SK, Okunishi K, Scott JP, Kumar Penke LR, Scruggs AM, Peters-Golden M (2013) Reversal of myofibroblast differentiation by prostaglandin E(2). Am J Respir Cell Mol Biol 48(5):550–558
Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, Hsi TC et al (2017) Regeneration of fat cells from myofibroblasts during wound healing. Science 355(6326):748–752
Klinger M, Marazzi M, Vigo D, Torre M (2008) Fat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reduction. Aesthet Plast Surg 32(3):465–469
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS (2007) Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 48(1):15–24
Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG (2015) Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther 6:145
Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444(7121):847–853
Kim EY, Kim WK, Oh KJ, Han BS, Lee SC, Bae KH (2015) Recent advances in proteomic studies of adipose tissues and adipocytes. Int J Mol Sci 16(3):4581–4599
Lehrke M, Lazar MA (2005) The many faces of PPARgamma. Cell 123(6):993–999
Ghosh AK, Bhattacharyya S, Lakos G, Chen SJ, Mori Y, Varga J (2004) Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum 50(4):1305–1318
Zheng F, Fornoni A, Elliot SJ, Guan Y, Breyer MD, Striker LJ, Striker GE (2002) Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am J Physiol Renal Physiol 282(4):F639–F648
Treindl F, Ruprecht B, Beiter Y, Schultz S, Döttinger A, Staebler A, Joos TO, Kling S, Poetz O, Fehm T (2016) A bead-based western for high-throughput cellular signal transduction analyses. Nat Commun 7:12852
Becker M, Maring JA, Schneider M, Herrera Martin AX, Seifert M, Klein O, Braun T, Falk V, Stamm C (2018) Towards a novel patch material for cardiac applications: tissue-specific extracellular matrix introduces essential key features to decellularized amniotic membrane. Int J Mol Sci 19(4):1032
Ojima K, Oe M, Nakajima I, Muroya S, Nishimura T (2016) Dynamics of protein secretion during adipocyte differentiation. FEBS Open Bio 6(8):816–826
Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I, Gravois A, Dow R, White A, Wu X et al (2007) Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics 6(1):18–28
Alvarez-Llamas G, Szalowska E, de Vries MP, Weening D, Landman K, Hoek A, Wolffenbuttel BHR, Roelofsen H, Vonk RJ (2007) Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics 6(4):589–600
Zhong J, Krawczyk SA, Chaerkady R, Huang H, Goel R, Bader JS, Wong GW, Corkey BE, Pandey A (2010) Temporal profiling of the secretome during adipogenesis in humans. J Proteome Res 9(10):5228–5238
Schaffler A, Buchler C (2007) Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells 25(4):818–827
Schmidt BA, Horsley V (2013) Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 140(7):1517–1527
Salathia NS, Shi J, Zhang J, Glynne RJ (2013) An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. J Invest Dermatol 133(3):812–821
Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J (2000) Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest 106(4):501–509
Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci V (2013) Autologous fat graft in scar treatment. J Craniofac Surg 24(5):1610–1615
Chevallet M, Diemer H, Van Dorssealer A, Villiers C, Rabilloud T (2007) Toward a better analysis of secreted proteins: the example of the myeloid cells secretome. Proteomics 7(11):1757–1770
Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC (2014) Adipose tissue-derived stromal cells inhibit TGF-beta1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg 134(4):699–712
Verhoekx JS, Mudera V, Walbeehm ET, Hovius SE (2013) Adipose-derived stem cells inhibit the contractile myofibroblast in Dupuytren’s disease. Plast Reconstr Surg 132(5):1139–1148
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, Yang X, He T, Guan H, Zheng Z et al (2016) Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res Ther 7(1):102
Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ (2005) PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288(6):L1146–L1153
Aoki Y, Maeno T, Aoyagi K, Ueno M, Aoki F, Aoki N, Nakagawa J, Sando Y, Shimizu Y, Suga T et al (2009) Pioglitazone, a peroxisome proliferator-activated receptor gamma ligand, suppresses bleomycin-induced acute lung injury and fibrosis. Respiration 77(3):311–319
Wei J, Ghosh AK, Sargent JL, Komura K, Wu M, Huang QQ, Jain M, Whitfield ML, Feghali-Bostwick C, Varga J (2010) PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One 5(11):e13778
Chou YT, Wang H, Chen Y, Danielpour D, Yang YC (2006) Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene 25(40):5547–5560
Tien ES, Davis JW, Vanden Heuvel JP (2004) Identification of the CREB-binding protein/p300-interacting protein CITED2 as a peroxisome proliferator-activated receptor alpha coregulator. J Biol Chem 279(23):24053–24063
Zhang C, Niu C, Yang K, Shaker A (2018) Human esophageal myofibroblast secretion of bone morphogenetic proteins and GREMLIN1 and paracrine regulation of squamous epithelial growth. Sci Rep 8(1):12354
McVicker BL, Bennett RG (2017) Novel anti-fibrotic therapies. Front Pharmacol 8:318
Shlyonsky V, Soussia IB, Naeije R, Mies F (2011) Opposing effects of bone morphogenetic protein-2 and endothelin-1 on lung fibroblast chloride currents. Am J Respir Cell Mol Biol 45(6):1154–1160
Pegorier S, Campbell GA, Kay AB, Lloyd CM (2010) Bone morphogenetic protein (BMP)-4 and BMP-7 regulate differentially transforming growth factor (TGF)-beta1 in normal human lung fibroblasts (NHLF). Respir Res 11:85
Ghosh AK, Vaughan DE (2012) PAI-1 in tissue fibrosis. J Cell Physiol 227(2):493–507
Schreiber I, Dorpholz G, Ott CE, Kragesteen B, Schanze N, Lee CT, Kohrle J, Mundlos S, Ruschke K, Knaus P (2017) BMPs as new insulin sensitizers: enhanced glucose uptake in mature 3T3-L1 adipocytes via PPARgamma and GLUT4 upregulation. Sci Rep 7(1):17192
Huang H, Song T-J, Li X, Hu L, He Q, Liu M, Lane MD, Tang Q-Q (2009) BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci 106(31):12670–12675
Hammarstedt A, Hedjazifar S, Jenndahl L, Gogg S, Grunberg J, Gustafson B, Klimcakova E, Stich V, Langin D, Laakso M et al (2013) WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc Natl Acad Sci U S A 110(7):2563–2568
Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, Ottmuller KJ, Mokhtari Z, Brede C, deRoos P et al (2018) Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Sci Immunol 3(19); doi: 10.1126/sciimmunol.aal2736
Acknowledgments
The authors thank Patrick Graff and Maria Thon for technical assistance. Moreover, the authors would like to acknowledge the support of Anja Briese and Dr. Christoph Sachse (NMI TT Pharmaservices).
Funding
This work was financially supported by the Einstein Center for Regenerative Therapies (ECRTs) and the Berlin-Brandenburg School for Regenerative Therapies (BSRT).
Author information
Authors and Affiliations
Contributions
K.H., G.E., and O.K. performed experiments. S.H., L.v.B. (at VUmc), and S.G. (at VUmc) supervised the work. K.H. and S.H. designed the experiments, analyzed the data, and wrote the manuscript. All the authors provided critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoerst, K., van den Broek, L., Sachse, C. et al. Regenerative potential of adipocytes in hypertrophic scars is mediated by myofibroblast reprogramming. J Mol Med 97, 761–775 (2019). https://doi.org/10.1007/s00109-019-01772-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01772-2