Abstract
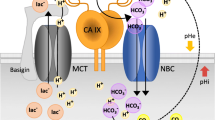
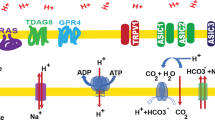
Among the players of the adaptive response of cancer cells able to promote a resistant and aggressive phenotype, carbonic anhydrase IX (CAIX) recently has emerged as one of the most relevant drug targets. Indeed, CAIX targeting has received a lot of interest, and selective inhibitors are currently under clinical trials. Hypoxia has been identified as the master inductor of CAIX, but, to date, very few is known about the influence that another important characteristic of tumor microenvironment, i.e., extracellular acidosis, exerts on CAIX expression and activity. In the last decades, acidic microenvironment has been associated with aggressive tumor phenotype endowed with epithelial-to-mesenchymal transition (EMT) profile, high invasive and migratory ability, apoptosis, and drug resistance. We demonstrated that melanoma, breast, and colorectal cancer cells transiently and chronically exposed to acidified medium (pH 6.7 ± 0.1) showed a significantly increased CAIX expression compared to those grown in standard conditions (pH 7.4 ± 0.1). Moreover, we observed that the CAIX inhibitor FC16-670A (also named SLC-0111, which just successfully ended phase I clinical trials) not only prevents such increased expression under acidosis but also promotes apoptotic and necrotic programs only in acidified cancer cells. Thus, CAIX could represent a selective target of acidic cancer cells and FC16-670A inhibitor as a useful tool to affect this aggressive subpopulation characterized by conventional therapy escape.
Key messages
-
Cancer cells overexpress CAIX under transient and chronic extracellular acidosis.
-
Acidosis-induced CAIX overexpression is NF-κB mediated and HIF-1α independent.
-
FC16-670A prevents CAIX overexpression and induces acidified cancer cell death.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Peppicelli S, Andreucci E, Ruzzolini J, Margheri F, Laurenzana A, Bianchini F, Calorini L (2017) Acidity of microenvironment as a further driver of tumor metabolic reprogramming. J Clin Cell Immunol 8:485
Damaghi M, Wojtkowiak JW, Gillies RJ (2013) pH sensing and regulation in cancer. Front Physiol 4:370
Neri D, Supuran CT (2011) Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10(10):767–777
Tafreshi NK, Lloyd MC, Bui MM, Gillies RJ, Morse DL (2014) Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Subcell Biochem 75:221–254
Thiry A, Dogné JM, Masereel B, Supuran CT (2006) Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 27(11):566–573
Zatovicova M, Sedlakova O, Svastova E, Ohradanova A, Ciampor F, Arribas J, Pastorek J, Pastorekova S (2005) Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br J Cancer 93(11):1267–1276
Shin HJ, Rho SB, Jung DC, Han IO, Oh ES, Kim JY (2011) Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci 124(Pt 7):1077–1087
Robertson N, Potter C, Harris AL (2004) Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res 64(17):6160–6165
Svastová E, Zilka N, Zat’ovicová M, Gibadulinová A, Ciampor F, Pastorek J, Pastoreková S (2003) Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with beta-catenin. Exp Cell Res 290(2):332–345
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J et al (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158(3):905–919
Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61(21):7992–7998
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60(24):7075–7083
De Simone G, Supuran CT (2010) Carbonic anhydrase IX: biochemical and crystallographic characterization of a novel antitumor target. Biochim Biophys Acta 1804(2):404–409
Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ (2011) Imaging pH and metastasis. NMR Biomed 24(6):582–591
Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK (2001) Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 61(16):6020–6024
Chiche J, Brahimi-Horn MC, Pouysségur J (2010) Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 14(4):771–794
Sørensen BS, Hao J, Overgaard J, Vorum H, Honoré B, Alsner J, Horsman MR (2005) Influence of oxygen concentration and pH on expression of hypoxia induced genes. Radiother Oncol 76(2):187–193
Sørensen BS, Alsner J, Overgaard J, Horsman MR (2007) Hypoxia induced expression of endogenous markers in vitro is highly influenced by pH. Radiother Oncol 83(3):362–366
Tang X, Lucas JE, Chen JL, LaMonte G, Wu J, Wang MC, Koumenis C, Chi JT (2012) Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer Res 72(2):491–502
Ihnatko R, Kubes M, Takacova M, Sedlakova O, Sedlak J, Pastorek J, Kopacek J, Pastorekova S (2006) Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int J Oncol 29(4):1025–1033
Peppicelli S, Bianchini F, Calorini L (2015) Dynamic scenario of metabolic pathway adaptation in tumors and therapeutic approach. Oncoscience 2(3):225–232
Peppicelli S, Toti A, Giannoni E, Bianchini F, Margheri F, Del Rosso M, Calorini L (2016) Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation. Cell Cycle 15(14):1908–1918
Matsubara T, Diresta GR, Kakunaga S, Li D, Healey JH (2013) Additive influence of extracellular pH, oxygen tension, and pressure on invasiveness and survival of human osteosarcoma cells. Front Oncol 3:199
Pacchiano F, Carta F, McDonald PC, Lou Y, Vullo D, Scozzafava A, Dedhar S, Supuran CT (2011) Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem 54(6):1896–1902
Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D et al (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 71(9):3364–3376
Carta F, Vullo D, Osman SM, AlOthman Z, Supuran CT (2017) Synthesis and carbonic anhydrase inhibition of a series of SLC-0111 analogs. Bioorg Med Chem 25(9):2569–2576
McCarty MF, Whitaker J (2010) Manipulating tumor acidification as a cancer treatment strategy. Altern Med Rev 15(3):264–272
Webb BA, Chimenti M, Jacobson MP, Barber DL (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11(9):671–677
Peppicelli S, Bianchini F, Calorini L (2014) Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev 33(2–3):823–832
Dubois L, Peeters S, Lieuwes NG, Geusens N, Thiry A, Wigfield S, Carta F, McIntyre A, Scozzafava A, Dogné JM et al (2011) Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol 99(3):424–431
Dubois L, Peeters SG, van Kuijk SJ, Yaromina A, Lieuwes NG, Saraya R, Biemans R, Rami M, Parvathaneni NK, Vullo D et al (2013) Targeting carbonic anhydrase IX by nitroimidazole based sulfamides enhances the therapeutic effect of tumor irradiation: a new concept of dual targeting drugs. Radiother Oncol 108(3):523–528
Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S (2014) Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol 4:400
Radvak P, Repic M, Svastova E, Takacova M, Csaderova L, Strnad H, Pastorek J, Pastorekova S, Kopacek J (2013) Suppression of carbonic anhydrase IX leads to aberrant focal adhesion and decreased invasion of tumor cells. Oncol Rep 29(3):1147–1153
Rofstad EK, Mathiesen B, Kindem K, Galappathi K (2006) Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 66(13):6699–6707
Peppicelli S, Bianchini F, Torre E, Calorini L (2014) Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin Exp Metastasis 31(4):423–433
Peppicelli S, Bianchini F, Calorini L (2015) Metabolic reprogramming as a continuous changing behavior of tumor cells. Tumour Biol 36(8):5759–5762
Wu H, Ying M, Hu X (2016) Lactic acidosis switches cancer cells from aerobic glycolysis back to dominant oxidative phosphorylation. Oncotarget 7(26):40621–40629
Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP et al (2002) Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 8(8):2595–2604
Simko V, Takacova M, Debreova M, Laposova K, Ondriskova-Panisova E, Pastorekova S, Csaderova L, Pastorek J (2016) Dexamethasone downregulates expression of carbonic anhydrase IX via HIF-1α and NF-κB-dependent mechanisms. Int J Oncol 49(4):1277–1288
Peppicelli S, Andreucci E, Ruzzolini J, Laurenzana A, Margheri F, Fibbi G, Del Rosso M, Bianchini F, Calorini L (2017) The acidic microenvironment as a possible niche of dormant tumor cells. Cell Mol Life Sci 74(15):2761–2771
Svastová E, Hulíková A, Rafajová M, Zat’ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J et al (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 577(3):439–445
Acknowledgements
This study was financially supported by grants from Istituto Toscano Tumori.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Andreucci, E., Peppicelli, S., Carta, F. et al. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J Mol Med 95, 1341–1353 (2017). https://doi.org/10.1007/s00109-017-1590-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1590-9
Keywords
Profiles
- Alessio Biagioni View author profile