Abstract
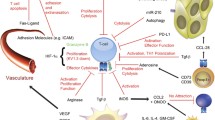
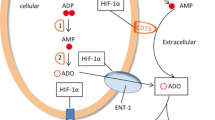
Intratumoral hypoxia and hypoxia inducible factor-1α (HIF-1-α)-dependent CD39/CD73 ectoenzymes may govern the accumulation of tumor-protecting extracellular adenosine and signaling through A2A adenosine receptors (A2AR) in tumor microenvironments (TME). Here, we explored the conceptually novel motivation to use supplemental oxygen as a treatment to inhibit the hypoxia/HIF-1α-CD39/CD73-driven accumulation of extracellular adenosine in the TME in order to weaken the tumor protection. We report that hyperoxic breathing (60 % O2) decreased the TME hypoxia, as well as levels of HIF-1α and downstream target proteins of HIF-1α in the TME according to proteomic studies in mice. Importantly, oxygenation also downregulated the expression of adenosine-generating ectoenzymes and significantly lowered levels of tumor-protecting extracellular adenosine in the TME. Using supplemental oxygen as a tool in studies of the TME, we also identified FHL-1 as a potentially useful marker for the conversion of hypoxic into normoxic TME. Hyperoxic breathing resulted in the upregulation of antigen-presenting MHC class I molecules on tumor cells and in the better recognition and increased susceptibility to killing by tumor-reactive cytotoxic T cells. Therapeutic breathing of 60 % oxygen resulted in the significant inhibition of growth of established B16.F10 melanoma tumors and prolonged survival of mice. Taken together, the data presented here provide proof-of principle for the therapeutic potential of systemic oxygenation to convert the hypoxic, adenosine-rich and tumor-protecting TME into a normoxic and extracellular adenosine-poor TME that, in turn, may facilitate tumor regression. We propose to explore the combination of supplemental oxygen with existing immunotherapies of cancer.
Key messages
-
Oxygenation decreases levels of tumor protecting hypoxia.
-
Oxygenation decreases levels of tumor protecting extracellular adenosine.
-
Oxygenation decreases expression of HIF-1alpha dependent tumor-protecting proteins.
-
Oxygenation increases MHC class I expression and enables tumor regression.





Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP (2002) Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110:993–1002
Eltzschig HK, Sitkovsky MV, Robson SC (2013) Purinergic signaling during inflammation. N Engl J Med 368:1260
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P et al (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 103(35):13132–13137
Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A (2008) Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res 14:5947–5952
Dewhirst MW, Cao Y, Moeller B (2008) Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8:425–437
Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R et al (2008) Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci U S A 105:19579–19586
Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL (2009) Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A 106:2353–2358
Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y (2011) Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia 13:206–216
Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ (2010) Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 107:1547–1552
Lukashev D, Ohta A, Sitkovsky M (2007) Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev 26:273–279
Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD (2012) Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother 61:917–926
Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B (2010) CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res 70:2245–2255
Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J (2013) CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 110:11091–11096
Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV (2005) Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol 3:e174
Raleigh JA, Dewhirst MW, Thrall DE (1996) Measuring tumor hypoxia. Semin Radiat Oncol 6:37–45
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL (2005) Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659–669
Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM (2006) Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1α, HIF-2α, and other pathways. J Biol Chem 281:15215–15226
Semenza GL (2014) Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76:39–56
Hubbi ME, Gilkes DM, Baek JH, Semenza GL (2012) Four-and-a-half LIM domain proteins inhibit transactivation by hypoxia-inducible factor 1. J Biol Chem 287:6139–6149
Shen C, Kaelin WG Jr (2013) The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol 23:18–25
Chida M, Voelkel NF (1996) Effects of acute and chronic hypoxia on rat lung cyclooxygenase. Am J Physiol 270:L872–878
Klein T, Shephard P, Kleinert H, Komhoff M (2007) Regulation of cyclooxygenase-2 expression by cyclic AMP. Biochim Biophys Acta 1773:1605–1618
Armstrong JM, Chen JF, Schwarzschild MA, Apasov S, Smith PT, Caldwell C, Chen P, Figler H, Sullivan G, Fink S et al (2001) Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene- deficient mice. Biochem J 354:123–130
Ohta A, Sitkovsky M (2001) Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414:916–920
Zhang B (2010) CD73: a novel target for cancer immunotherapy. Cancer Res 70:6407–6411
Busk M, Horsman MR (2013) Relevance of hypoxia in radiation oncology: pathophysiology, tumor biology and implications for treatment. Q J Nucl Med Mol Imaging 57:219–234
Margaretten NC, Witschi H (1988) Effects of hyperoxia on growth characteristics of metastatic murine tumors in the lung. Cancer Res 48:2779–2783
Ren J, Mi Z, Stewart NA, Jackson EK (2009) Identification and quantification of 2',3'-cAMP release by the kidney. J Pharmacol Exp Ther 328:855–865
Longhi MS, Robson SC, Bernstein SH, Serra S, Deaglio S (2013) Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med (Berl) 91:165–172
Huang Y, Goel S, Duda DG, Fukumura D, Jain RK (2013) Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 73:2943–2948
Ohta A, Sitkovsky M (2006) Caveats in promising therapeutic targeting of the anti-inflammatory A2 adenosine receptors: the notes of caution. Discovery, Nature Reviews Drug
Aggarwal NR, D'Alessio FR, Eto Y, Chau E, Avalos C, Waickman AT, Garibaldi BT, Mock JR, Files DC, Sidhaye V et al (2013) Macrophage A2A adenosinergic receptor modulates oxygen-induced augmentation of murine lung injury. Am J Respir Cell Mol Biol 48:635–646
Nowak-Machen M, Schmelzle M, Hanidziar D, Junger W, Exley M, Otterbein L, Wu Y, Csizmadia E, Doherty G, Sitkovsky M et al (2013) Pulmonary natural killer T cells play an essential role in mediating hyperoxic acute lung injury. Am J Respir Cell Mol Biol 48:601–609
Sitkovsky MV, Paul WE (1988) Immunology. Global or directed exocytosis? Nature 332:306–307
Acknowledgments
This work was supported by the funding from Northeastern University and NIH grants (PI: M.S.) R01 CA-111985; R01 GM-097320; R01 CA-112561; R21 AT 002788. We thank Dr. Richard Marsh, an expert in the field of energetics, kinematics, and kinetics for assistance with monitoring gas compositions in hyperoxia units.
Conflict of interest
The authors declare they have no competing interests as defined by Molecular Medicine or other interests that might be perceived to influence the results and discussion reported in this paper.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2,396 kb)
Rights and permissions
About this article
Cite this article
Hatfield, S.M., Kjaergaard, J., Lukashev, D. et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1α-dependent and extracellular adenosine-mediated tumor protection. J Mol Med 92, 1283–1292 (2014). https://doi.org/10.1007/s00109-014-1189-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1189-3