Abstract
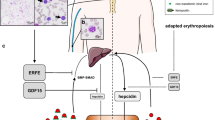
Congenital dyserythropoietic anaemias (CDAs) are heterogeneous, hereditary disorders hallmarked by ineffective erythropoiesis and tissue iron overload. Growth differentiation factor 15 (GDF15) was suggested to mediate iron overload in iron-loading anaemias, such as the thalassaemias and CDAI by suppressing hepcidin, the key regulator of iron absorption. Here, we show that serum GDF15 concentrations are elevated in subjects with CDAI and CDAII. Despite similar disease characteristics, CDAI patients present with significantly higher GDF15 concentrations compared to CDAII patients. Hepcidin concentrations are inappropriately low in CDAII patients considering the severe hepatic iron overload associated with this disorder. GDF15 significantly correlates with the degree of anaemia (Hb), the response of erythropoiesis (reticulocyte index) as well as with iron availability in the serum (transferrin saturation). The observation that GDF15 is elevated in CDAII patients is consistent with the proposal that GDF15 is among the erythroid factors down-regulating hepcidin and contributing to iron overload in conditions of dyserythropoiesis.


Similar content being viewed by others
References
Wickramasinghe SN, Wood WG (2005) Advances in the understanding of the congenital dyserythropoietic anaemias. Br J Haematol 131:431–446
Dgany O, Avidan N, Delaunay J, Krasnov T, Shalmon L, Shalev H, Eidelitz-Markus T, Kapelushnik J, Cattan D, Pariente A, Tulliez M, Cretien A, Schischmanoff PO, Iolascon A, Fibach E, Koren A, Rossler J, Le Merrer M, Yaniv I, Zaizov R, Ben-Asher E, Olender T, Lancet D, Beckmann JS, Tamary H (2002) Congenital dyserythropoietic anemia type I is caused by mutations in codanin-1. Am J Hum Genet 71:1467–1474
Iolascon A, D'Agostaro G, Perrotta S, Izzo P, Tavano R, Miraglia del Giudice B (1996) Congenital dyserythropoietic anemia type II: molecular basis and clinical aspects. Haematologica 81:543–559
Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, Chen W, Paw BH, Hopfner KP, Holzmann K, Russo R, Esposito MR, Spano D, De Falco L, Heinrich K, Joggerst B, Rojewski MT, Perrotta S, Denecke J, Pannicke U, Delaunay J, Pepperkok R, Heimpel H (2009) Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Gen 41:936–940
Andrews NC (2008) Forging a field: the golden age of iron biology. Blood 112:219–230
Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S (2010) Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica 95:501–504. doi:10.3324/haematol.2009.014399
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL (2007) High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med 13:1096–1101
Tamary H, Shalev H, Perez-Avraham G, Zoldan M, Levi I, Swinkels DW, Tanno T, Miller JL (2008) Elevated growth differentiation factor 15 expression in patients with congenital dyserythropoietic anemia type I. Blood 112:5241–5244
Walter PB, Harmatz P, Vichinsky E (2009) Iron metabolism and iron chelation in sickle cell disease. Acta Haematol 122:174–183
Finkenstedt A, Bianchi P, Theurl I, Vogel W, Witcher DR, Wroblewski VJ, Murphy AT, Zanella A, Zoller H (2009) Regulation of iron metabolism through GDF15 and hepcidin in pyruvate kinase deficiency. Br J Haematol 144:789–793
Heimpel H, Anselstetter V, Chrobak L, Denecke J, Einsiedler B, Gallmeier K, Griesshammer A, Marquardt T, Janka-Schaub G, Kron M, Kohne E (2003) Congenital dyserythropoietic anemia type II: epidemiology, clinical appearance, and prognosis based on long-term observation. Blood 102:4576–4581. doi:10.1182/blood-2003-02-0613
Heimpel H, Schwarz K, Ebnother M, Goede JS, Heydrich D, Kamp T, Plaumann L, Rath B, Roessler J, Schildknecht O, Schmid M, Wuillemin W, Einsiedler B, Leichtle R, Tamary H, Kohne E (2006) Congenital dyserythropoietic anemia type I (CDA I): molecular genetics, clinical appearance, and prognosis based on long-term observation. Blood 107:334–340
Kroot JJ, Laarakkers CM, Geurts-Moespot AJ, Grebenchtchikov N, Pickkers P, van Ede AE, Peters HP, van Dongen-Lases E, Wetzels JF, Sweep FC, Tjalsma H, Swinkels DW (2010) Immunochemical and mass-spectrometry-based serum hepcidin assays for iron metabolism disorders. Clin Chem 56:1570–1579. doi:10.1373/clinchem.2010.149187
Suominen P, Punnonen K, Rajamaki A, Majuri R, Hanninen V, Irjala K (1999) Automated immunoturbidimetric method for measuring serum transferrin receptor. Clin Chem 45:1302–1305
Vernet M, Doyen C (2000) Assessment of iron status with a new fully automated assay for transferrin receptor in human serum. Clin Chem Lab Med 38:437–442. doi:10.1515/CCLM.2000.064
Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C (2005) Hepcidin is decreased in TFR2 hemochromatosis. Blood 105:1803–1806
Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E (2007) Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica 92:583–588
Kemna EH, Kartikasari AE, van Tits LJ, Pickkers P, Tjalsma H, Swinkels DW (2008) Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis 40:339–346. doi:10.1016/j.bcmd.2007.10.002
Flanagan JM, Peng H, Wang L, Gelbart T, Lee P, Johnson Sasu B, Beutler E (2006) Soluble transferrin receptor-1 levels in mice do not affect iron absorption. Acta Haematol 116:249–254. doi:10.1159/000095875
Lakhal S, Talbot NP, Crosby A, Stoepker C, Townsend AR, Robbins PA, Pugh CW, Ratcliffe PJ, Mole DR (2009) Regulation of growth differentiation factor 15 expression by intracellular iron. Blood 113:1555–1563
Theurl I, Finkenstedt A, Schroll A, Nairz M, Sonnweber T, Bellmann-Weiler R, Theurl M, Seifert M, Wroblewski VJ, Murphy AT, Witcher D, Zoller H, Weiss G (2010) Growth differentiation factor 15 in anaemia of chronic disease, iron deficiency anaemia and mixed type anaemia. Br J Haematol 148:449–455. doi:10.1111/j.1365-2141.2009.07961.x
Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, Paulson RF, Miller JL (2009) Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood 114:181–186. doi:10.1182/blood-2008-12-195503
Pinto JP, Ribeiro S, Pontes H, Thowfeequ S, Tosh D, Carvalho F, Porto G (2008) Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPalpha. Blood 111:5727–5733. doi:10.1182/blood-2007-08-106195
Mleczko-Sanecka K, Casanovas G, Ragab A, Breitkopf K, Muller A, Boutros M, Dooley S, Hentze MW, Muckenthaler MU (2010) SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood 115:2657–2665
Acknowledgements
We thank Rosi Leichtle (Department for Internal Medicine III, University Hospital of Ulm) for the collection of patient samples, and Raeka S. Aiyar and Julien Gagneur for their useful advice during the analysis of the data. M.U.M. acknowledges funding from the E-RARE/BMBF project 01GM1005.
Conflicts of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
G. Casanovas and D. W. Swinkels contributed equally to this work.
H. Heimpel and M. U. Muckenthaler contributed equally to this work.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Soluble transferrin receptor correlations in CDAII patients. Correlations of soluble transferrin receptor with (A) ferritin, (B) the hepcidin to ferritin ratio and (C) hepcidin in CDAII patients (n = 23). Correlations are shown on a logarithmic scale, with the statistical values (ρ and P) indicated. Trend lines are based on the logarithms of the values. (JPEG 218 kb)
Supplementary Table 1
Serum parameters tested in healthy volunteers (1–39) and unaffected family members of CDA patients (40–48). *NA not available (PDF 65 kb)
Supplementary Table 2
Representative blood parameters tested in CDAI and CDAII patients. (*) History of splenectomy; (**) iron depletion; (***) regular transfusions. NA not available (PDF 69 kb)
Supplementary Table 3
Blood parameters tested in samples from CDAI and CDAII patients. (*) History of splenectomy; (**) receiving iron depletion; (***) regular transfusions. NA not available (PDF 73 kb)
Supplementary Table 4
Serum parameters in healthy controls, CDAI and CDAII patients. Mean values and rank (highest and lowest value) are shown. Statistical significance was calculated by a nonparametric Mann–Whitney test. * (number of men/number of women); NS not significant; † reference values are shown for controls (measurements not available). (PDF 57 kb)
Rights and permissions
About this article
Cite this article
Casanovas, G., Swinkels, D.W., Altamura, S. et al. Growth differentiation factor 15 in patients with congenital dyserythropoietic anaemia (CDA) type II. J Mol Med 89, 811–816 (2011). https://doi.org/10.1007/s00109-011-0751-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-011-0751-5