Abstract
Drainage of central nervous system (CNS) antigens to the brain-draining cervical lymph nodes (CLN) is likely crucial in the initiation and control of autoimmune responses during multiple sclerosis (MS). We demonstrate neuronal antigens within CLN of MS patients. In monkeys and mice with experimental autoimmune encephalomyelitis (EAE) and in mouse models with non-inflammatory CNS damage, the type and extent of CNS damage was associated with the frequencies of CNS antigens within the cervical lymph nodes. In addition, CNS antigens drained to the spinal-cord-draining lumbar lymph nodes. In human MS CLN, neuronal antigens were present in pro-inflammatory antigen-presenting cells (APC), whereas the majority of myelin-containing cells were anti-inflammatory. This may reflect a different origin of the cells or different drainage mechanisms. Indeed, neuronal antigen-containing cells in human CLN did not express the lymph node homing receptor CCR7, whereas myelin antigen-containing cells in situ and in vitro did. Nevertheless, CLN from EAE-affected CCR7-deficient mice contained equal amounts of myelin and neuronal antigens as wild-type mice. We conclude that the type and frequencies of CNS antigens within the CLN are determined by the type and extent of CNS damage. Furthermore, the presence of myelin and neuronal antigens in functionally distinct APC populations within MS CLN suggests that differential immune responses can be evoked.
Similar content being viewed by others
Introduction
Irreversible neuronal damage is a major pathological feature of multiple sclerosis (MS) and ranges from mild pathology to complete axonal transection [1, 2]. The cause of neuronal damage is not yet elucidated, but autoreactive B and T cells directed against neuronal antigens could conceivably be instrumental [3]. Indeed, MS patients have increased circulating antibody levels against the neuronal proteins neurofilament light (NF-L) and neurofilament heavy (NF-H) [4–6] in serum and against the medium subunit of neurofilament in cerebrospinal fluid (CSF) [7]. In addition, T cells from MS patients proliferate in response to the neuronal antigens synapsin and neuron-specific enolase (NSE) [8, 9]. In mice, T-cell-mediated autoimmunity against neuronal antigens leads to central nervous system (CNS) inflammation and experimental autoimmune encephalomyelitis (EAE) symptoms [10–14].
Autoreactive lymphocytes against myelin proteins and likely also against neuronal antigens are recruited into the CNS [2]. Where in the body these lymphocytes initially are activated is still unclear. This is a critical issue, in view of possible therapeutic interventions aiming to limit activation of autoreactive T cells. Under experimental conditions, dendritic cells activate naïve CD4+ transgenic T cells directed against proteolipid protein (PLP)139-151 within the CNS [15]. However, the classical view on the initiation of naïve T cell activation holds that antigens or antigen-containing antigen-presenting cells (APC) must be transferred to the brain-draining cervical lymph nodes (CLN) to effectively activate naïve T cells [16, 17]. CNS-resident APC subsequently reactivate these antigen-experienced T cells and allow them to exert their effector functions within the target organ [18, 19]. A crucial role of the CLN in CNS inflammation is supported by the observation that surgical removal of the CLN reduced the number of brain lesions in cryolesion-enhanced EAE in the rat [20]. Furthermore, myelin basic protein, PLP, and neutral lipid-containing APC are present in the CLN of MS patients and EAE-affected marmoset and rhesus monkeys [21, 22]. These APC stimulated myelin-specific T cell proliferation [21], demonstrating that myelin drainage to the CLN during demyelinating disease results in the activation of autoreactive T cells.
We hypothesize that drainage of neuronal antigens from the target organ to the CLN may be similarly involved in initiating or modulating neuron-specific immune responses. We have analyzed whether brain-derived neuronal antigens are present in CLN of MS patients and of selected animal models with different degrees of CNS damage. In addition, we determined whether these antigens are present in distinct APC subsets, which may influence T cell activation.
Materials and methods
MS tissues
Human jugular (deep) CLN and supraclavicular CLN were taken from MS patients with active disease at autopsy. Chronic inactive and active plaques were present in the cerebrum of these patients. The MS patients died of non-MS-related causes. In addition, CLN were taken from controls without neurological disease at autopsy by the Netherlands Brain Bank (coordinator Dr. R. Ravid). CLN were snap-frozen in liquid nitrogen and stored at −80°C until use.
EAE tissues
All animal studies in the current study followed the principles of animal care and were approved by local ethical committees based on national legislation. Tissues were obtained from animals that were used for studies designed for other purposes, thus avoiding the sacrifice of animals for the present study only.
Deep CLN were isolated from rhesus monkeys (Macaca mulatta) with acute EAE (n = 5) which was induced as described [23] and from rhesus monkeys with collagen-induced arthritis (n = 3) [24]. Deep CLN were also isolated from common marmoset monkeys (Callithrix jacchus) during chronic EAE (n = 5) [25, 26]. Control CLN were from marmosets (n = 2) immunized with ovalbumin in complete Freund’s adjuvant (CFA) [21]. The monkeys were housed at the Biomedical Primate Research Center (BPRC, Rijswijk, the Netherlands).
Deep and superficial CLN were isolated from Biozzi ABH (H-2dq1) mice, obtained from stock bred at the BPRC, at the acute phase of disease (n = 3) and during the first relapse (n = 3). EAE was induced by immunization with spinal cord homogenate or myelin oligodendrocyte glycoprotein (MOG)8-21 in CFA as described [27, 28]. Deep and superficial CLN were also isolated from CCR7-deficient (n = 4) and wild-type mice (n = 4) [29] with chronic EAE, obtained from stock bred at the animal facility of the Max Delbrück Center for Molecular Medicine (Berlin, Germany). Chronic EAE was induced by s.c. injections of 200 μg MOG35-55 emulsified in CFA (Difco Laboratories) containing 400 μg of Mycobacterium tuberculosis. Mice received i.v. injections with 200 ng Bordetella pertussis toxin (Sigma) on days 0 and 2 postimmunization. The CCR7-deficient mice used in these experiments had been backcrossed to C57BL/6 mice for eight generations.
All animals were examined daily for clinical symptoms of EAE as described [21, 25, 28, 30]. Tissues were snap-frozen in liquid nitrogen and stored at −80°C.
Non-inflammatory CNS damage models
Ischemia was induced in C57BL/6 mice (BgVV) by occlusion of the left middle cerebral artery (MCAO) [31]. The MCAO did not exceed 60 min. CLN were isolated 24 h (n = 5) and 72 h (n = 5) after MCAO. Entorhinal cortex lesion (ECL) and facial nerve axotomy (FNA) were performed as described [32] in C57BL/6 mice (n = 5 and n = 3, respectively; Charles River). CLN were isolated 7 days later.
Demyelination was induced chemically by cuprizone (Sigma-Aldrich) in SJL/J mice (n = 3; Janvier) and C57BL/6 mice (n = 3; Harlan) as described [33]. These animals were representative animals within a larger experiment designed for other purposes, consisting of 18 SJL/J mice and 18 C57BL/6 mice. Five to 6 weeks after start of treatment with cuprizone, superficial CLN, deep CLN, and lumbar lymph nodes (LLN) were isolated. All tissues were snap-frozen and stored at −80°C.
Immunohistochemistry
Immunohistochemistry was performed as described [34, 35]. Primary antibodies were polyclonal rabbit antibodies against transcription growth factor (TGF)-β (Santa Cruz) and NF-L (Abcam), and monoclonal mouse antibodies against NSE (MIG M3; Abcam), microtubule-associated protein-2 (MAP-2; HSM 5; Pierce Biotechnology), NF-H (SMI-32; Sternberger Monoclonals), PLP (J1/06 [36]), HLA-DP/DQ/DR (CR3/43; DAKO), CD40 (5D12; dr. M. de Boer), interleukin 1 receptor antagonist (IL-1ra; A71B6D11; Bioscource), tumor necrosis factor (TNF)-α (61E71; U Cytech), IL-12p40/p70 (C8.6; BD), and CCR7 (2H4; BD).
Primary antibodies were detected by biotinylated donkey anti-rabbit immunoglobulin (Ig; Amersham) or rabbit anti-mouse Ig (DAKO) and horseradish peroxidase (HRP)-conjugated avidin–biotin complex (DAKO). HRP activity was revealed with 3-amino-9-ethylcarbazole (Sigma-Aldrich), which resulted in a red precipitate. Brain or spinal cord sections from the same species were used as positive control tissue. As negative controls, sections were incubated with isotype-matched primary antibodies of irrelevant specificity, or the primary antibody was omitted.
Immunofluorescence
Double labeling was performed using immunofluorescence as described [37]. Sections were incubated with primary antibodies for 1 h at room temperature (RT), followed by fluorescein isothiocyanate (FITC)- or tetramethylrhodamine isothiocyanate (TRITC)-labeled rabbit anti-mouse Ig (DAKO) or TRITC-labeled swine anti-rabbit Ig (DAKO) for 30 min at RT. Subsequently, sections were incubated for 1 h at RT with Alexa594-labeled anti-MAP-2 (IgG1 Zenon labeling kit, Molecular Probes) or with anti-MOG for 1 h at RT, followed by biotinylated anti-IgG2a (SBA) for 30 min at RT and FITC-labeled streptavidin (DAKO) for 1 h at RT. Sections were mounted in glycerol–Tris–4’,6-diamidino-2-phenylindole (DAPI; 50 μg/ml; Molecular Probes).
As controls, single stainings were performed following the procedure described above but with omission of one of the primary antibodies used in the double staining. Human reactive tonsil or brain was used as positive control tissue. Negative controls are described above.
CCR7 expression by myelin-laden human monocyte-derived macrophages
Monocytes from healthy donors were routinely purified and cultured [38]. For the current study, experiments were performed with cells from two individual donors that responded representatively for at least 50 donors assessed in the preceding years. Monocyte-derived macrophages were seeded into 24-well tissue culture plates at 2 × 105 cells per well. After 24 h, non-adherent cells were removed and the remaining cells were incubated with human myelin [39] for the indicated times. Control macrophages were obtained from the same donor.
Total mRNA was extracted from cell cultures as described [38]. CCR7 mRNA expression was analyzed by real-time polymerase chain reaction using the iCycler (Bio-rad) and the iQ SYBR Green supermix (Bio-rad). The housekeeping gene HPRT1 was used for normalization. The following primers were used: 5′-TGGTCGTGGTCTTCATAGTC-3′ and 5′-CAGGTGCTACTGGTGATGTT-3′ for CCR7 and 5′-TGACACTGGCAAAACAATGCA-3′ and 5′-AGCTTGCTGGTGAAAAGGACC-3′ for HPRT1.
CCR7 surface expression by human monocyte-derived macrophages was determined using flow cytometry. Fc receptors were blocked using 20% Fc-block (Miltenyi Biotec) and CCR7 was stained using phycoerythrin-labeled mouse anti-CCR7 (150503; R&D Systems) for 30 min on ice. An isotype-matched antibody of irrelevant specificity was used as negative control. Samples were analyzed on a FACSCalibur flow cytometer using CellQuest analysis software (Becton Dickinson).
T cell proliferation assay
Deep CLN, superficial CLN, and LLN were isolated from C57BL/6 mice with chronic EAE and from Biozzi ABH mice with relapsing–remitting EAE. EAE in C57BL/6 mice was induced by immunization with 200 μg MOG35-55 in CFA [30] and in Biozzi ABH mice as described above. Lymph nodes from five to ten EAE-affected animals were pooled per experiment to obtain enough cells for restimulation. A single cell suspension was obtained by passing the lymph nodes through a 70-μm gauze. Lymph node cells, 2 × 105, were seeded into 96-well round-bottomed plates (Nunc) and stimulated with the indicated concentrations of MOG8-21, MOG35-55 (both from Advanced Biotechnology Center, Imperial College London), recombinant mouse NF-L (rmNF-L) [40], or ovalbumin (Worthington). After 4 days, T cell proliferation was determined by incorporation of [3H]-thymidine for 18 h (Amersham Biosciences) as described [30].
Image analysis
The area of the sections was measured using a VIDAS-RT image analysis system (Kontron Elektronic/Carl Zeiss) to obtain the number of antigen-positive cells per square millimeter. Differences in number of neuronal antigen-containing cells in CLN between treatment groups were determined by a two-tailed Mann–Whitney test using the statistical software program SPSS, version 11.0. A significance level of 0.05 was used.
Results
Presence of neuronal antigens within the CLN of MS patients
To determine the presence of neuronal antigens in CLN of MS patients with active disease, cryosections were stained with antibodies specific for three neuronal antigens: MAP-2, NSE, and NF-H. Cells containing each of the neuronal antigens were detectable in CLN from all MS patients, which were predominantly large cells located in the medulla of the lymph node with a morphology resembling macrophages. Neuronal antigen-containing cells were also found in the paracortex, where dendritic cells and T cells are located (Fig. 1a–c).
Neuronal antigens drain to the CLN in MS. CLN of MS patients contain cells that are positive for the neuronal antigens NSE (a), MAP-2 (b), and NF-H (c). Scale bars 20 μm; insert 5 μm. Quantification of the number of MAP-2 (d), NSE (e), and NF-H (f) positive cells demonstrated a higher, but not significantly different, number of neuronal antigen-containing cells in CLN of three MS patients than in CLN of three controls without neurological disease. Three sections from CLN of each patient were quantified for each neuronal antigen. Results are shown as box plots with medians, 25th and 75th percentiles as boxes, tenth and 90th percentiles as whiskers
As previously described, neuronal antigen-containing cells were also present in CLN of control subjects (Fig. 1d–f) [41, 42]. To investigate whether the presence of neuronal antigens in CLN of MS patients was the result of neuronal damage in the CNS or the result of expression by cells in the CLN, neuronal antigen-containing cells were quantified and compared with the number of neuronal antigen-containing cells in CLN of control subjects without neurological disease. As the numbers of MAP-2-, NSE-, and NF-H-positive cells in CLN of MS patients and control subjects were not statistically different (Fig. 1d–f), we could not definitively conclude that increased neuronal damage within the CNS resulted in increased neuronal antigen drainage to CLN. Hence, we performed an elaborate analysis in a variety of EAE models and in animal models of non-inflammatory CNS damage (Table 1).
Presence of neuronal antigen-containing cells in CLN of EAE-affected animals reflects the intensity of neuronal damage
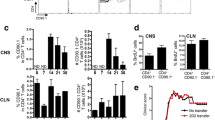
It has been well established that the rhMOG-induced EAE model in common marmoset monkeys is characterized by chronic progressive disease with limited axonal destruction, whereas the same EAE model in rhesus monkeys follows a short-lasting acute disease course with extensive axonal destruction [43]. In CLN of EAE-affected marmoset monkeys, NSE- and NF-H-containing cells were observed (Fig. 2a,b). Quantification revealed a higher, but not significantly different, number of neuronal antigen-containing cells in CLN of EAE-affected marmosets as compared to CFA controls (Fig. 2c,d). In contrast, the CLN of EAE-affected rhesus monkeys contained significantly more MAP-2 and NF-H-containing cells (Fig. 2e,f), than the CLN of CFA controls (p < 0.05; Fig. 2g,h).
The presence of neuronal antigen-containing cells during EAE reflects the extent of neuronal damage within the CNS. CLN of EAE-affected common marmoset monkeys contain NSE (a) and NF-H (b) positive cells. More NSE (c) and NF-H (d) positive cells were observed in CLN of five marmosets with EAE as compared to two CFA-immunized control marmosets, although this difference was not significant. In CLN of EAE-affected rhesus monkeys, MAP-2 (e) and NF-H (f) positive cells were found. Quantification of MAP-2 (g) and NF-H (h) positive cells revealed a significantly higher number of cells in CLN of five rhesus monkeys with EAE than in CLN of three collagen-induced arthritis (CIA) control monkeys (*p < 0.05). NF-L-positive cells were present in the deep CLN of EAE Biozzi ABH mice (i). Deep CLN from three animals with acute disease as well as from three mice in the first relapse contained significantly more NF-L-positive cells than CLN from three CFA-immunized control mice (j; *p < 0.05). EAE scores from one representative animal out of three are shown (k). Three sections from CLN of each monkey and four sections from CLN of each mouse were quantified for each neuronal antigen. Results are given as box plots as described in Fig. 1 with extreme values as filled circles. Scale bars 10 μm
The neuronal antigen NF-L was detected in deep CLN from EAE-affected Biozzi ABH mice with clinical EAE, but only rarely in superficial CLN (Fig. 2i). This EAE model is characterized by a first peak of acute disease with inflammation and axonal injury, followed by one or more relapses. As an example, Fig. 2k shows the typical relapsing–remitting disease course in this model. CLN isolated during acute EAE as well as during the first relapse had significantly more NF-L-containing cells than CFA-immunized control mice (p < 0.05; Fig. 2j). We observed similar numbers of NF-L-containing cells in the first EAE episode and the subsequent relapse.
As in CLN of MS patients, neuronal antigen-containing cells in the CLN of EAE-affected mice and monkeys were large macrophage-like cells which were located in the medulla of the lymph node and, although in lower numbers, in the paracortex.
Drainage of CNS antigens to the CNS-draining lymph nodes is reflected by the extent and type of CNS damage
To further assess whether the extent and also the type of CNS insult determines drainage of CNS compounds and to investigate drainage routes of CNS antigens in mice, we used different mouse models with non-inflammatory CNS damage. The MCAO model is characterized by massive ischemic lesions in the cortex, striatum, and the hippocampus [31]. Superficial as well as deep CLN isolated 24 h after MCAO contained numerous PLP- and NF-L-containing cells (Fig. 3b,c). This number was reduced after 72 h (Fig. 3b,c), indicating rapid and transient drainage following CNS damage. In CLN isolated from mice with ECL and FNA, few PLP- and NF-L-containing cells were observed in the CLN (Fig. 3b,c), reflecting the mild CNS damage in these models [44, 45]. The cuprizone model is characterized by extensive CNS demyelination induced by cuprizone, which selectively kills oligodendrocytes [33]. Little drainage of the myelin antigen PLP to the superficial CLN was noticed (Fig. 3a, b). In contrast, the deep CLN of both SJL/J mice (Fig. 3a,c) as well as C57BL/6 mice (data not shown) contained significantly higher numbers of PLP-containing cells (p < 0.001). We also detected myelin antigens in the LLN (Fig. 3a,c), reflecting drainage of antigens from the spinal cord. Only few NF-L-containing cells were observed in the CLN of cuprizone-treated mice (Fig 3b,c), which is in line with the limited neuronal damage in this model [46].
The type and extent of non-inflammatory CNS damage correlates with the frequencies of CNS antigens in the CNS-draining lymph nodes. PLP-containing cells in the superficial CLN, deep CLN, and the spinal cord-draining LLN of cuprizone-treated SJL/J mice. Scale bars 50 μm (a). Quantification of the number of PLP- and NF-L-containing cells in the superficial CLN (b) and deep CLN (c). Tissues were isolated 24 h (n = 5) and 72 h (n = 5) after MCAO, 7 days after ECL (n = 5), 7 days after FNA (n = 3), and after 6 weeks of cuprizone treatment (Cup; n = 3). Cells were quantified in at least two sections of each tissue. Data represent the mean number of positive cells ± SEM. *p < 0.05
Neuronal antigen-containing cells have an APC phenotype
Although the majority of neuronal antigen-containing cells in MS CLN were located in the medulla of the lymph node, such cells were also found in the paracortex (Fig. 1a–c), where APC interact with naïve T lymphocytes. We therefore investigated whether neuronal antigen-containing cells in MS CLN express APC markers. As expected in a secondary lymphoid organ, numerous cells in the CLN expressed major histocompatibility complex (MHC) class II and CD40 of which only a fraction contained the neuronal antigen MAP-2. However, almost all (90–100%) MAP-2-positive cells expressed MHC class II (Fig. 4a) and the costimulatory molecule CD40 (Fig. 4b). This indicates that neuronal antigen-containing cells are APC, such as dendritic cells or macrophages, which may be involved in the induction of autoimmunity against neuronal antigens or conversely in the control of autoreactivity.
MAP-2-containing cells in human MS CLN are immunocompetent APC. Immunofluorescent MAP-2 staining in human MS CLN (red) and staining for the APC markers MHC class II (green; a) and CD40 (green; b). Overlay shows coexpression of MAP-2 with MHC class II and CD40, which was observed in 90% to 100% of the MAP-2-positive cells. Nuclei are stained with DAPI (blue). Stainings were performed on two sections of CLN from three different MS patients. Scale bars 5 μm
T cells in CLN proliferate to MOG peptide, without evident epitope spreading to NF-L
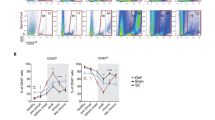
The localization of neuronal antigens in APC within the CLN may activate autoreactive T cells. This was tested in three independent experiments. Two experiments were using C57BL/6 mice in which EAE was induced by injection with MOG35-55 (n = 5–10 per experiment). One additional experiment was using Biozzi ABH mice (n = 10) in which EAE was induced by injection with MOG8-21. Figure 5 shows a representative experiment, in which we observed dose-dependent T cell proliferation against the immunizing peptide MOG35-55 in deep CLN, superficial CLN as well as in LLN. CLN and LLN from mice immunized with MOG8-21 demonstrated quantitatively similar proliferation against MOG8-21 (data not shown). No proliferation against the irrelevant control antigen OVA was seen. We did not detect NF-L-specific T cell proliferation in the two EAE models used for these experiments.
Drainage of neuronal antigens to CLN in MOG-peptide-induced EAE does not elicit a detectable NF-L-specific T cell response. Stimulation of lymph node cells from deep CLN, superficial CLN, and LLN from EAE-affected C57BL/6 and Biozzi ABH mice with rmNF-L, MOG35-55, and ovalbumin demonstrates a dose-dependent proliferation against the myelin-derived peptide MOG, whereas no proliferation was observed against NF-L and ovalbumin. Lymph nodes of five to ten EAE-affected mice were pooled per experiment to obtain enough cells for restimulation. Results are presented as mean with the standard deviation of triplicates and are representative for three independent experiments using two different MOG-peptide-induced EAE models
Differential expression of pro- and anti-inflammatory molecules by myelin-containing versus neuronal antigen-containing cells
Myelin-laden myeloid cells in MS brain with foamy appearance demonstrate a strong anti-inflammatory phenotype in situ and in vitro, implying a possible role in the resolution of local inflammation during MS [38]. To assess whether this also holds true for myelin antigen-containing cells and neuronal antigen-containing cells in CLN of MS patients, we determined the expression of pro- and anti-inflammatory molecules. The majority of MOG-containing cells (90–100%) coexpressed the anti-inflammatory molecules IL-1ra (Fig. 6a) and TGF-β (Fig. 6b), whereas only a minority of the cells (2–30%) expressed the pro-inflammatory molecule IL-12p40/p70, indicating that most MOG-containing cells display an anti-inflammatory phenotype (Table 2). In contrast, none of the MAP-2-containing cells expressed IL-1ra and TGF-β, while the majority of the cells (86–100%) expressed IL-12p40/p70 (Fig. 6c) and TNF-α (Fig. 6d), indicating a pro-inflammatory phenotype (Table 2). These data were paralleled in the CLN of EAE-affected rhesus monkeys, where 50–95% of the MOG-containing cells expressed IL-1ra and 13–47% expressed IL-12p40/p70, whereas 75–94% of the MAP-2-containing cells expressed IL-12p40/p70 and none expressed IL-1ra.
Differential expression of pro- and anti-inflammatory molecules by MAP-2- and MOG-containing cells in human MS CLN. Immunofluorescent labeling of MOG (green) and the anti-inflammatory molecules IL-1ra (red; a) and TGF-β (red; b) and immunofluorescent labeling of MAP-2 (red) and of the pro-inflammatory molecules IL-12p40/p70 (green; c), and TNF-α (green; d) in human MS CLN. The overlays show colocalization of MOG and the anti-inflammatory molecules IL-1ra and TGF-β, which was observed in 90% to 100% of the MOG-positive cells, and colocalization of MAP-2 and the pro-inflammatory molecules IL-12p40/p70 and TNF-α, which was observed in 86% to 100% of the MAP-2-positive cells. Nuclei are stained with DAPI (blue). Results are representative for two sections of CLN from an MS patient for double stainings of MOG with TGF-β and TNF-α and of MAP-2 with TGF-β and for at least two sections of CLN from three different MS patients for double stainings of MAP-2 with IL-12p40/p70, TNF-α, and IL-1ra and double stainings of MOG with IL-1ra and IL-12p40/p70. Scale bars 5 μm
Part of MOG-containing cells in CLN of MS patients express CCR7, whereas MAP-2-containing cells do not express CCR7
Drainage of CNS antigens to the CLN may either occur as soluble proteins or within phagocytes [16, 47]. We have previously shown that myelin antigen-containing cells in rhesus monkey CLN express the chemokine receptor CCR7 [21], which mediates leukocyte homing to draining lymph nodes. Similarly, we observed that 20–42% of MOG-containing cells in human MS CLN expressed CCR7, whereas none of the MAP-2-containing cells expressed this marker (Table 2). These data were paralleled in the CLN of EAE-affected rhesus monkeys, in which 20–46% of MOG-containing cells expressed CCR7, and MAP-2-containing cells did not express CCR7.
To investigate whether CCR7 expression is induced by the uptake of myelin antigens, we incubated human monocyte-derived macrophages from healthy donors in vitro with human myelin and determined CCR7 mRNA expression and CCR7 surface protein expression. We observed a time-dependent increase of CCR7 mRNA expression after myelin ingestion. As compared to control macrophages, myelin-laden macrophages demonstrated a 38.4 ± 4.2-fold increase in CCR7 mRNA expression after 24 h of myelin ingestion, which was reduced to a 6.3 ± 0.9-fold increase after 7 days of myelin ingestion. In addition to mRNA, CCR7 surface protein expression was also increased after myelin ingestion (data not shown).
Finally, we assessed whether CCR7 expression affects the drainage of CNS antigens to CLN. To this end, we compared the number of CNS antigen-containing cells in the deep and superficial CLN of EAE-affected CCR7-deficient and wild-type mice. In our hands, CCR7-deficient mice developed mild EAE and CLN of these mice were therefore compared to CLN from wild-type mice with comparable EAE symptoms. Unexpectedly, the numbers of both myelin and neuronal antigen-containing cells in CLN of EAE-affected CCR7-deficient mice were not statistically different as compared to wild-type mice (Table 3).
Discussion
Interaction between the brain and the secondary lymphoid organs allows regulation of immune responses in the brain [48]. This is exemplified by the crucial role of CLN in brain lesion expansion in cryolesion-enhanced EAE in rats [20] and by the presence of myelin antigens in APC in CLN of MS patients and EAE-affected rhesus monkeys and common marmoset monkeys [21, 22]. The current study assessed whether neuronal antigens also drain to the CLN after neuronal damage in the CNS.
MS is both clinically and pathologically a heterogeneous disease demonstrating various degrees of neuronal damage. The obtained CLN tissues were therefore from patients at various disease stages. To further determine drainage in a more controlled setting with defined disease stages, we used a variety of EAE models and models of non-inflammatory CNS insult, representing different degrees of neuronal damage. We report here that neuronal antigens are present in CLN of MS patients and of animals after induction of EAE, MCAO, ECL, and FNA. In rhesus monkeys and Biozzi ABH mice, animals in which EAE results in significant neuronal damage within the CNS, the quantity of neuronal antigen-containing cells in the CLN reflected the intensity of neuronal damage in the CNS. Furthermore, qualitative and quantitative drainage of CNS antigens followed the type and extent of CNS damage in mice after MCAO, ECL, FNA, and cuprizone treatment. Interestingly, the frequency of CNS antigens in the CLN was high 24 h after MCAO and reduced 72 h after MCAO, indicating that drainage occurs rapidly but transient following CNS damage. This transient CNS drainage might also cause the few CNS antigen-containing cells in the CLN of the ECL and FNA model, which were isolated 7 days after CNS damage induction. These data extend our previous data and demonstrate that, following CNS damage, both myelin and neuronal antigens drain to CLN.
The presence of neuronal antigen-containing cells in CLN of control subjects was not unexpected and may be due to innervation of the CLN [49], expression of neuronal antigens by the cells themselves [41, 42], or to aging. The frequency of neuronal antigen-containing cells in CLN from control subjects seems to differ between species, which might be due to differential neuronal turnover rates between species or differential expression levels of neuronal antigens by the cells.
To investigate drainage routes from the brain to the CLN, we used different non-inflammatory CNS damage models [31, 33, 44–46]. In cuprizone-treated animals, numerous cells containing myelin antigens were observed in the deep CLN, whereas such cells were observed only occasionally in the superficial CLN. We have found the same drainage route in EAE-affected mice, where we observed high numbers of neuronal antigen-containing cells in the deep CLN but hardly in the superficial CLN. These data indicate that CNS antigens preferentially drain to the deep CLN. In contrast, both the deep CLN as well as the superficial CLN of MCAO-treated mice contained numerous CNS antigens. This may be caused by a different drainage route as the result of the massive CNS damage or by the fact that this massive CNS damage has destroyed (part of) the drainage route. Myelin antigens were also observed in the LLN of cuprizone-treated mice, which are likely derived from the spinal cord, in which demyelination also takes place as is the case in MS and EAE. In fact, whereas CLN are crucial in brain lesion expansion in cryolesion-induced EAE in rats, they have no effect on lesion expansion in the lumbar part of the spinal cord [20], suggesting distinct functional relationships between CNS compartments and their local draining lymph nodes.
The nature of immune responses against CNS antigens in the CLN might be dictated by the functional phenotype of the CNS antigen-containing cells. We therefore determined the phenotype of myelin and neuronal antigen-containing cells in human MS CLN. Neuronal antigen-containing cells in MS CLN expressed the APC markers MHC class II and CD40, indicating that these cells are equipped for antigen presentation to T lymphocytes. Furthermore, neuronal antigen-containing cells were observed in the paracortex of the lymph node, where APC encounter and activate naïve T cells. Indeed, T cell responses against neuronal antigens have been demonstrated in MS patients and EAE-affected animals [8–10], and T cell-mediated EAE symptoms can be induced by immunization with neuronal antigens [11, 13, 14]. Despite this, we did not detect T cell proliferation against NF-L in CLN from EAE mice immunized with MOG35-55 or MOG8-21. The lack of T cell proliferation we consistently found might be due to the absence of intermolecular spreading or to the fact that the draining antigens may act immunosuppressively on proliferation within the draining lymph nodes.
The current study shows that phagocytes containing the neuronal antigen MAP-2 in CLN of MS patients and EAE-affected rhesus monkeys had a pro-inflammatory phenotype. In contrast, the majority of phagocytes containing the myelin antigen MOG demonstrated an anti-inflammatory phenotype, suggesting a relation with the anti-inflammatory myelin-laden foamy macrophages present within MS lesions [38]. This differential expression of pro- and anti-inflammatory molecules likely influences the type of immune response against these antigens. The difference in functional phenotype between MAP-2 and MOG-containing cells may be the consequence of the inflammatory status of the microenvironment in which the cells have taken up their antigens. Alternatively, the nature of the phagocytosed antigen may direct the immunophenotype of the cell into a pro- or anti-inflammatory mode of action.
As both myelin and neuronal antigens are found in CLN, the question arises how these antigens reach these lymph nodes. The two likely mechanisms are either as soluble antigen or by active transport within phagocytosing cells [16, 17], such as the anti-inflammatory foamy macrophages within MS lesions [38]. Since anti-inflammatory macrophages express the lymph node homing receptor CCR7 [50], we determined whether a CCR7-dependent mechanism could be involved. This study shows that, similar to APC in MS brain as well as MOG-containing cells in EAE-affected rhesus CLN [21, 51], myelin-containing cells in human MS CLN and in vitro express CCR7, but MAP-2-containing cells do not. CLN from EAE-affected CCR7-deficient mice contain slightly more myelin and neuronal antigens as compared to CLN from EAE-affected wild-type mice, indicating that CCR7 is not necessarily involved. This strongly suggests that either other chemokine receptors are able to guide cell migration to the CLN or that CNS antigens drain as soluble antigens through CSF to the CLN. This hypothesis is supported by increased free neuronal proteins in CSF of MS patients and EAE mice as compared to healthy controls [52, 53].
In conclusion, we here report that neuronal antigen-containing cells are present in CLN during MS and in various animal models for CNS damage. The frequencies of these cells correlated with the extent of neuronal damage. In addition, neuronal antigen-containing cells in human MS CLN are present in functionally different APC subsets as compared to the majority of myelin antigen-containing APC. The presence of neuronal antigens in APC with a pro-inflammatory phenotype and of myelin antigens in APC with an anti-inflammatory phenotype points at a different potential to activate functionally distinct T cell subsets.
Abbreviations
- APC:
-
antigen-presenting cell
- CFA:
-
complete Freund’s adjuvant
- CLN:
-
cervical lymph node
- CNS:
-
central nervous system
- CSF:
-
cerebrospinal fluid
- EAE:
-
experimental autoimmune encephalomyelitis
- ECL:
-
entorhinal cortex lesion
- FNA:
-
facial nerve axotomy
- IL-1ra:
-
IL-1 receptor antagonist
- LLN:
-
lumbar lymph nodes
- MAP-2:
-
microtubule-associated protein-2
- MCAO:
-
middle cerebral artery occlusion
- MHC:
-
major histocompatibility complex
- MOG:
-
myelin oligodendrocyte glycoprotein
- MS:
-
multiple sclerosis
- NF-L:
-
neurofilament light
- NF-H:
-
neurofilament heavy
- NSE:
-
neuron-specific enolase
References
Ferguson B, Matyszak MK, Esiri MM, Perry VH (1997) Axonal damage in acute multiple sclerosis lesions. Brain 120:393–399
Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747
Huizinga R, Linington C, Amor S (2008) Resistance is futile: antineuronal autoimmunity in multiple sclerosis. Trends Immunol 29:54–60
Silber E, Semra YK, Gregson NA, Sharief MK (2002) Patients with progressive multiple sclerosis have elevated antibodies to neurofilament subunit. Neurology 58:1372–1381
Eikelenboom MJ, Petzold A, Lazeron RH, Silber E, Sharief M, Thompson EJ, Barkhof F, Giovannoni G, Polman CH, Uitdehaag BM (2003) Multiple sclerosis: neurofilament light chain antibodies are correlated to cerebral atrophy. Neurology 60:219–223
Bartoš A, Fialova L, Soukupova J, Kukal J, Malbohan I, Pit’ha J (2007) Antibodies against light neurofilaments in multiple sclerosis patients. Acta Neurol Scand 116:100–107
Bartoš A, Fialova L, Soukupova J, Kukal J, Malbohan I, Pit’ha J (2007) Elevated intrathecal antibodies against the medium neurofilament subunit in multiple sclerosis. J Neurol 254:20–25
Polak T, Schlaf G, Scholl U, Krome-Cesar C, Mader M, Felgenhauer K, Weber F (2001) Characterization of the human T cell response against the neuronal protein synapsin in patients with multiple sclerosis. J Neuroimmunol 115:176–181
Forooghian F, Cheung RK, Smith WC, O’Connor P, Dosch HM (2007) Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol 27:388–396
Furlan R, Brambilla E, Sanvito F, Roccatagliata L, Olivieri S, Bergami A, Pluchino S, Uccelli A, Comi G, Martino G (2003) Vaccination with amyloid-beta peptide induces autoimmune encephalomyelitis in C57/BL6 mice. Brain 126:285–291
Mor F, Quintana F, Mimran A, Cohen IR (2003) Autoimmune encephalomyelitis and uveitis induced by T cell immunity to self beta-synuclein. J Immunol 170:628–634
Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Topphoff US, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F (2005) Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron 46:421–432
Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, Abramsky O (2006) Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol 63:1459–1467
Huizinga R, Heijmans N, Schubert P, Gschmeissner S, ‘t Hart BA, Herrmann H, Amor S (2007) Immunization with neurofilament light protein induces spastic paresis and axonal degeneration in Biozzi ABH mice. J Neuropathol Exp Neurol 66:295–304
McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD (2005) Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11:335–339
Karman J, Ling C, Sandor M, Fabry Z (2004) Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol 173:2353–2361
Mohindru M, Kang B, Kim BS (2004) Functional maturation of proteolipid protein(139–151)-specific Th1 cells in the central nervous system in experimental autoimmune encephalomyelitis. J Neuroimmunol 155:127–135
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11:146–152
Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11:328–334
Phillips MJ, Needham M, Weller RO (1997) Role of cervical lymph nodes in autoimmune encephalomyelitis in the Lewis rat. J Pathol 182:457–464
de Vos AF, van Meurs M, Brok HP, Boven LA, Hintzen RQ, van der Valk P, Ravid R, Rensing S, Boon L, ‘t Hart BA, Laman JD (2002) Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol 169:5415–5423
Fabriek BO, Zwemmer JN, Teunissen CE, Dijkstra CD, Polman CH, Laman JD, Castelijns JA (2005) In vivo detection of myelin proteins in cervical lymph nodes of MS patients using ultrasound-guided fine-needle aspiration cytology. J Neuroimmunol 161:190–194
Kerlero de Rosbo N, Brok HP, Bauer J, Kaye JF, ‘t Hart BA, Ben-Nun A (2000) Rhesus monkeys are highly susceptible to experimental autoimmune encephalomyelitis induced by myelin oligodendrocyte glycoprotein: characterisation of immunodominant T- and B-cell epitopes. J Neuroimmunol 110:83–96
de Bakker NP, van Erck MG, Otting N, Lardy NM, Noort RC, ‘t Hart BA, Jonker M, Bontrop RE (1992) Resistance to collagen-induced arthritis in a nonhuman primate species maps to the major histocompatibility complex class I region. J Exp Med 175:933–937
‘t Hart BA, Bauer J, Muller HJ, Melchers B, Nicolay K, Brok H, Bontrop RE, Lassmann H, Massacesi L (1998) Histopathological characterization of magnetic resonance imaging-detectable brain white matter lesions in a primate model of multiple sclerosis: a correlative study in the experimental autoimmune encephalomyelitis model in common marmosets (Callithrix jacchus). Am J Pathol 153:649–663
Brok HP, Uccelli A, Kerlero De Rosbo N, Bontrop RE, Roccatagliata L, de Groot NG, Capello E, Laman JD, Nicolay K, Mancardi GL, Ben-Nun A, Hart BA (2000) Myelin/oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis in common marmosets: the encephalitogenic T cell epitope pMOG24-36 is presented by a monomorphic MHC class II molecule. J Immunol 165:1093–1101
Baker D, O’Neill JK, Gschmeissner SE, Wilcox CE, Butter C, Turk JL (1990) Induction of chronic relapsing experimental allergic encephalomyelitis in Biozzi mice. J Neuroimmunol 28:261–270
Amor S, Baker D, Groome N, Turk JL (1993) Identification of a major encephalitogenic epitope of proteolipid protein (residues 56–70) for the induction of experimental allergic encephalomyelitis in Biozzi AB/H and nonobese diabetic mice. J Immunol 150:5666–5672
Höpken UE, Droese J, Li JP, Joergensen J, Breitfeld D, Zerwes HG, Lipp M (2004) The chemokine receptor CCR7 controls lymph node-dependent cytotoxic T cell priming in alloimmune responses. Eur J Immunol 34:461–470
Visser L, Jan de Heer H, Boven LA, van Riel D, van Meurs M, Melief MJ, Zahringer U, van Strijp J, Lambrecht BN, Nieuwenhuis EE, Laman JD (2005) Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol 174:808–816
Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A (2003) Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 198:725–736
Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac AD, Gerard C, Boddeke HW, Nitsch R, Kettenmann H (2004) CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci 24:8500–8509
Hiremath MM, Saito Y, Knapp GW, Ting JP, Suzuki K, Matsushima GK (1998) Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol 92:38–49
Laman JD, van Meurs M, Schellekens MM, de Boer M, Melchers B, Massacesi L, Lassmann H, Claassen E, ‘t Hart BA (1998) Expression of accessory molecules and cytokines in acute EAE in marmoset monkeys (Callithrix jacchus). J Neuroimmunol 86:30–45
Claassen E, Jeurissen SHM (1996) A step by step guide to in situ immune response analysis of lymphoid tissues by immunohistochemical methods, Weir’s handbook of experimental immunology. Blackwell Science, Cambridge
Laman JD, Visser L, Maassen CB, de Groot CJ, de Jong LA, ‘t Hart BA, van Meurs M, Schellekens MM (2001) Novel monoclonal antibodies against proteolipid protein peptide 139–151 demonstrate demyelination and myelin uptake by macrophages in MS and marmoset EAE lesions. J Neuroimmunol 119:124–130
Visser L, Melief MJ, van Riel D, van Meurs M, Sick EA, Inamura S, Bajramovic JJ, Amor S, Hintzen RQ, Boven LA, t Hart BA, Laman JD (2006) Phagocytes containing a disease-promoting Toll-like receptor/Nod ligand are present in the brain during demyelinating disease in primates. Am J Pathol 169:1671–1685
Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD (2006) Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129:517–526
Norton WT, Poduslo SE (1973) Myelination in rat brain: method of myelin isolation. J Neurochem 21:749–757
Heins S, Wong PC, Muller S, Goldie K, Cleveland DW, Aebi U (1993) The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J Cell Biol 123:1517–1533
Yan WH, Cao MD, Liu JR, Xu Y, Han XF, Xing Y, Wang JZ (2005) Effects of EGF and bFGF on expression of microtubule-associated protein tau and MAP-2 mRNA in human umbilical cord mononuclear cells. Cell Biol Int 29:153–157
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 174:11–20
‘t Hart BA, Bauer J, Brok HP, Amor S (2005) Non-human primate models of experimental autoimmune encephalomyelitis: variations on a theme. J Neuroimmunol 168:1–12
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Kwidzinski E, Mutlu LK, Kovac AD, Bunse J, Goldmann J, Mahlo J, Aktas O, Zipp F, Kamradt T, Nitsch R, Bechmann I (2003) Self-tolerance in the immune privileged CNS: lessons from the entorhinal cortex lesion model. J Neural Transm Suppl 65:29–49
Irvine KA, Blakemore WF (2006) Age increases axon loss associated with primary demyelination in cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol 175:69–76
Weller RO (1998) Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J Neuropathol Exp Neurol 57:885–894
Cserr HF, Knopf PM (1992) Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13:507–512
Bellinger DL, Lorton D, Lubahn C, Felten DL (2001) Innervation of lymphoid organs-association of nerves with cells of the immune system and their implications in disease. In: Ader R, Cohen N, Felten D (eds) Psychoneuroimmunology. Academic, New York, pp 55–111
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311
Kivisakk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM (2004) Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol 55:627–638
Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J (2003) Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 61:1720–1725
Norgren N, Edelstam A, Stigbrand T (2005) Cerebrospinal fluid levels of neurofilament light in chronic experimental autoimmune encephalomyelitis. Brain Res Bull 67:264–268
Amor S, Smith PA, Hart B, Baker D (2005) Biozzi mice: of mice and human neurological diseases. J Neuroimmunol 165:1–10
Tsunoda I, Tanaka T, Terry EJ, Fujinami RS (2007) Contrasting roles for axonal degeneration in an autoimmune versus viral model of multiple sclerosis: when can axonal injury be beneficial? Am J Pathol 170:214–226
Bechmann I, Peter S, Beyer M, Gimsa U, Nitsch R (2001) Presence of B7–2 (CD86) and lack of B7–1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo. FASEB J 15:1086–1088
Moran LB, Graeber MB (2004) The facial nerve axotomy model. Brain Res Brain Res Rev 44:154–178
Acknowledgements
The authors thank the Netherlands Brain Bank (coordinator Dr. R. Ravid) and the UK Multiple Sclerosis Tissue Bank Charing Cross Campus for supplying brain tissue, Dr. Christiane Nolte for providing FNA tissues, and T. van Os for microphotographs. This work was supported by grants of the Dutch MS Research Foundation (MS-01-471, MS-01-457, MS-00-417, and MS-02-490).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors JDL and LAB share senior authorship.
The authors have no conflict of interest.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Zwam, M., Huizinga, R., Melief, MJ. et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med 87, 273–286 (2009). https://doi.org/10.1007/s00109-008-0421-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-008-0421-4