Abstract
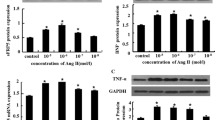
Elevations in angiotensin II (AngII) and transforming growth factor (TGF-β1) levels are often found under conditions leading to progression of heart failure. From several studies, it is evident that AngII enhances TGF-β1 expression via activator protein 1 (AP-1) activation, and that this pathway is involved in hypertrophic growth of the heart muscle and in the development of cardiac fibrosis. We now continued characterization of the signaling pathway stimulated by AngII in ventricular cardiomyocytes of rat and analyzed if the enhancement of TGF-β1 expression by AngII may also contribute to apoptosis induction, which is another predictor of heart failure progression. Stimulation of cardiomyocytes with 100 nM AngII for 2 h activated the transcription factors AP-1 and GATA by 68.6±23.9 or 70.7±9.8%. Induction of both factors was mediated by p38 mitogen-activated protein kinase (MAPK) because it was totally blocked using a specific inhibitor of the kinase (SB202190). When GATA was inhibited by transformation of cardiomyocytes with decoy oligonucleotides, AngII could not enhance TGF-β1 expression. This inhibition was observed on the mRNA level in real-time polymerase chain reaction and on the protein level in Western blots. As a consequence, upon AngII stimulation for 24 h, release of TGF-β1 from cardiomyocytes was also reduced from 240.5±50.4 to 130.5±22.1% (p<0.05). In contrast to the early induction of GATA and AP-1, the transcription factor similar to mothers against decapentaplegic homolog (SMAD) was induced by AngII after 24 h. This stimulation was dependent on TGF-β1 because it was blocked by antibodies specific for TGF-β1. Twenty-four hours after AngII addition, the number of apoptotic cardiomyocytes increased by 6.5±1.2%, and this apoptosis induction was blocked when SMAD activity was inhibited by transformation of cardiomyocytes with SMAD decoy oligonucleotides. In conclusion, the transcription factors AP-1 and GATA are activated by p38 MAPK upon AngII stimulation, and both are needed to enhance TGF-β1 expression in ventricular cardiomyocytes. TGF-β1 acts in an autocrine loop on the cells to induce apoptosis via SMAD signaling. Thus, the often-found correlation between AngII, TGF-β1, AP-1, and SMAD in pathogenesis of heart disease reflects the proapoptotic signaling pathway induced by AngII in cardiomyocytes.







Similar content being viewed by others
References
Peffer JM, Pfeffer MA (1988) Angiotensin converting enzyme inhibition and ventricular remodeling in heart failure. Am J Med 84:37–44
Pinto YM, Pinto-Sietsma S-J, Philipp T, Engler S, Koßmehl P, Hocher B, Marquardt H, Sethmann S, Lauster R, Merker H-J, Paul M (2000) Reduction in left ventricular messenger RNA for transforming growth factor beta(1) attenuates left ventricular fibrosis and improves survival without lowering blood pressure in the hypertensive TGR(mRen2)27 Rat. Hypertension 36:747–754
Kim S, Yoshiyama M, Izumi Y, Kawano H, Kimoto M, Zhan Y, Iwao H (2001) Effects of combination of ACE inhibitor and angiotensin receptor blocker on cardiac remodeling, cardiac function, and survival in rat heart failure. Circulation 103:148–154
The SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325:293–302
Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 327:669–677
Pitt B, Poole-Wilson P, Segal R, Martinez F, Dickstein K, Camm A, Konstam M, Riegger G, Klinger G, Neaton J (2000) Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the Losartan Heart Failure Survival Study ELITE II. Lancet 355:1582–1587
Villarreal FJ, MacKenna DA, Omens JH, Dillmann WH (1995) Myocardial remodeling in hypertensive Ren-2 transgenic rats. Hypertension 25:98–104
Diep QN, El Mabrouk M, Yue P, Schiffrin EL (2002) Effect of AT1 receptor blockade on cardiac apoptosis in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 282:1635–1641
Ravassa S, Fortuño MA, González A, López B, Zalba G, Fortuño A, Díez J (2000) Mechanisms of increased susceptibility to angiotensin II-induced apoptosis in ventricular cardiomyocytes of spontaneously hypertensive rats. Hypertension 36:1065–1071
Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM (1993) Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res 72:1245–1254
Bouzegrhane F, Thibault G (2002) Is angiotensin II a proliferative factor of cardiac fibroblasts? Cardiovasc Res 53:304–312
Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P (1997) Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 29:859–870
Cigola E, Kajstura J, Li B, Meggs LG, Anversa P (1997) Angiotensin II activates programmed myocyte cell death in vitro. Exp Cell Res 231:363–371
Ruf S, Piper HM, Schluter KD (2002) Specific role for the extracellular signal-regulated kinase pathway in angiotensin II- but not phenylephrine-induced cardiac hypertrophy in vitro. Pflugers Arch 443:483–490
Schluter KD, Zhou XJ, Piper HM (1995) Induction of hypertrophic responsiveness to isoproterenol by TGF-beta in adult rat cardiomyocytes. Am J Physiol 269:C1311–C1316
Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schlüter KD, Böhm M (2002) Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol 283:H1253–H1262
Wenzel S, Taimor G, Piper HM, Sschlüter KD (2001) Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-beta expression in adult ventricular cardiomyocytes. FASEB J 15:2291–2293
Taimor G, Schlüter K-D, Best P, Helmig S, Piper HM (2004) Transcription activator protein 1 mediates alpha- but not beta-adrenergic hypertrophic growth responses in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 286:H2369–H2375
Schneiders D, Heger J, Best P, Piper HM, Taimor G (2005) SMAD proteins are involved in apoptosis induction in ventricular cardiomyocytes. Cardiovasc Res 67:87–96
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228
Pikkarainen S, Tokola H, Majalahti-Palviainen T, Kerkelä R, Hautala N, Bhalla SS, Charron F, Nemer M, Vuolteenaho O, Ruskoaho H (2003) GATA-4 is a nuclear mediator of mechanical stretch-activated hypertrophic program. J Biol Chem 278:23807–23816
Molkentin JD (2000) The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem 275:38949–38952
Moriguchi Y, Matsubara H, Mori Y, Murasawa S, Masaki H, Maruyama K, Tsutsumi Y, Shibasaki Y, Tanaka Y, Nakajima T, Oda K, Iwasaka T (1999) Angiotensin II-induced transactivation of epidermal growth factor receptor regulates fibronectin and transforming growth factor-beta synthesis via transcriptional and posttranscriptional mechanisms. Circ Res 84:1073–1084
Heimer R, Bashey RI, Kyle J, Jimenez SA (1995) TGF-beta modulates the synthesis of proteoglycans by myocardial fibroblasts in culture. J Mol Cell Cardiol 27:2191–2198
Dixon IM, Hao J, Reid NL, Roth JC (2000) Effect of chronic AT(1) receptor blockade on cardiac Smad overexpression in hereditary cardiomyopathic hamsters. Cardiovasc Res 46:286–297
Hayakawa Y, Chandra M, Miao W, Shirani J, Heller Brown J, Dorn GW II, Armstrong RC, Kitsis RN (2003) Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation 108:3036–3041
Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN (2003) A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 111:1497–1504
Piper HM, Probst I, Hütter JF, Spiekermann PG (1982) Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol 14:397–412
Moniotte S, Vaerman JL, Kockx MM, Larrouy D, Langin D, Noirhomme P, Balligand JL (2001) Real-time RT-PCR for the detection of beta-adrenoceptor messenger RNAs in small human endomyocardial biopsies. J Mol Cell Cardiol 33:2121–2133
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Taimor G, Rakow A, Piper HM (2001) Transcription activator protein 1 (AP-1) mediates NO-induced apoptosis of adult cardiomyocytes. FASEB J 15:2518–2520
Taimor G, Schluter K, Piper HM (2001) Hypertrophy-associated gene induction after beta-adrenergic stimulation in adult cardiomyocytes. J Mol Cell Cardiol 33:503–511
Godfrey K (1985) Comparing the means of several groups. N Engl J Med 313:1450–1456
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105
Omura T, Yoshiyama M, Yoshida K, Nakamura Y, Kim S, Iwao H, Takeuchi K, Yoshikawa J (2002) Dominant negative mutant of c-Jun inhibits cardiomyocyte hypertrophy induced by endothelin 1 and phenylephrine. Hypertension 39:81–86
Jain J, McCaffrey PG, Miner Z, Kerppola TK, Lambert JN, Verdine GL, Curran T, Rao A (1993) The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 365:352–355
Macián F, García-Rodríguez C, Rao A (2000) Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J 19:4783–4795
Herzig TC, Jobe SM, Aoki H, Molkentin JD, Cowley AW, Izumo S, Markham BE (1997) Angiotensin II type1a receptor gene expression in the heart: AP-1 and GATA-4 participate in the response to pressure overload. Proc Natl Acad Sci USA 94:7543–7548
Xia Y, McMillin JB, Lewis A, Moore M, Zhu WG, Williams RS, Kellems RE (2000) Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways and up-regulates the adenylosuccinate synthetase 1 gene. J Biol Chem 275:1855–1863
Yaar R, Cataldo LM, Tzatsos A, Francis CE, Zhao Z, Ravid K (2002) Regulation of the A3 adenosine receptor gene in vascular smooth muscle cells: role of a cAMP and GATA element. Mol Pharmacol 62:1167–1176
Chinenov Y, Kerppola TK (2001) Close encounters of many kinds: Fos–Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438–2452
Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE (1998) Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1:611–617
Acknowledgments
The authors thank Daniela Schreiber, Sergej Kechter, and Birgit Störr for excellent technical assistance. This study was supported by the Philip Morris Grant (Pi 162/11-3) and by the Deutsche Forschungsgemeinschaft (TA 216/2-1). It is part of the thesis submitted by Dunja Schröder.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schröder, D., Heger, J., Piper, H.M. et al. Angiotensin II stimulates apoptosis via TGF-β1 signaling in ventricular cardiomyocytes of rat. J Mol Med 84, 975–983 (2006). https://doi.org/10.1007/s00109-006-0090-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-006-0090-0