Abstract
Purpose
To report our results of computed tomography-guided interstitial high-dose-rate (HDR) brachytherapy (BRT) in the treatment of patients with recurrent inoperable glioblastoma multiforme (GBM).
Patients and methods
Between 1995 and 2014, 135 patients were treated with interstitial HDR BRT for inoperable recurrent GBM located within previously irradiated volumes. Patient’s median age was 57.1 years (14–82 years). All patients were pretreated with surgery, postoperative external beam radiation therapy (EBRT) and systemic chemotherapy (ChT). The median recurrent tumor volume was 42 cm3 (2–207 cm3). The prescribed HDR dose was median 40 Gy (30–50 Gy) delivered in twice-daily fractions of 5.0 Gy over consecutive days. No repeat surgery or ChT was administered in conjunction with BRT. Survival from BRT, progression-free survival (PFS), toxicity as well as the impact of several prognostic factors were evaluated.
Results
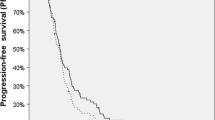
At a median follow-up of 9.2 months, the median overall survival following BRT and the median PFS were 9.2 and 4.6 months, respectively. Of the prognostic variables evaluated in univariate analysis, extent of surgery at initial diagnosis, tumor volume at recurrence, as well as time from EBRT to BRT reached statistical significance, retained also in multivariate analysis. Eight patients (5.9%) developed treatment-associated complications including intracerebral bleeding in 4 patients (2.9%), symptomatic focal radionecrosis in 3 patients (2.2%), and severe convulsion in 1 patient (0.7%).
Conclusions
For patients with recurrent GBM, interstitial HDR BRT is an effective re-irradiation method for even larger tumors providing palliation without excessive toxicity.
Zusammenfassung
Ziel
Vorstellung der CT(Computertomographie)-gestützten interstitiellen HDR(„high dose rate“)-Brachytherapie (BRT) zur Behandlung inoperabler GBM(Glioblastoma-multiforme)-Rezidive.
Patienten und Methoden
Von 1995–2014 wurden insgesamt 135 Patienten mit inoperablen GBM-Rezidiven mittels interstitieller BRT rebestrahlt. Das mediane Patientenalter betrug 57,1 Jahre (14–82 Jahre). Alle Patienten waren voroperiert und hatten im Rahmen ihrer Primärbehandlung eine adjuvante Radiochemotherapie erhalten. In der Rezidivsituation betrug das mediane Tumorvolumen 42 cm3 (2–207 cm3). Die Rebestrahlung erfolgte als fraktionierte interstitielle HDR-BRT mit einer medianen Gesamtdosis von 40 Gy (30–50 Gy), appliziert in 2‑mal täglichen Fraktionsdosen zu jeweils 5 Gy über aufeinander folgende Tage. Kein Patient erhielt eine simultane Chemotherapie oder eine erneute Resektion nach BRT. Evaluiert wurden das Überleben nach BRT, das Progressionsfreie Überleben, das Toxizitätsprofil sowie der Einfluss prognostischer Faktoren.
Ergebnisse
Bei einer medianen Nachbeobachtungszeit von 9,2 Monaten betrugen das mediane Überleben nach BRT und das mediane progressionsfreie Überleben jeweils 9,2 und 4,6 Monate. Als statistisch signifikant hinsichtlich der evaluierten prognostischen Faktoren erwiesen sich das Resektionsausmaß bei der Primärbehandlung, das Tumorvolumen in der Rezidivsituation und die Zeit zwischen adjuvanter Radiotherapie und BRT sowohl in der univariaten als auch in der multivariaten Analyse. Acht (5,9 %) therapieassoziierte Komplikationen wurden verzeichnet: intrakranielle Blutungen bei 4 (2,9 %), symptomatische Radionekrosen in 3 (2,2 %) und schwere konvulsive Episoden bei einem (0,7 %) Patienten.
Schlussfolgerung
Die CT-gestützte interstitielle HDR-BRT ist eine sichere Methode für die Rebestrahlung insbesondere größerer GBM-Rezidive, die ohne exzessive Toxizität Palliation bieten kann.



Similar content being viewed by others
References
Stupp R, van den Bent MWP, Martin J et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Wick W, Platten M, Meisner C et al (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–715
Laws ER, Shaffrey ME (1999) The inherent invasiveness of cerebral gliomas: implications for clinical management. Int J Dev Neurosci 17:413–420
Straube C, Elpula G, Gempt J et al (2017) Re-irradiation after gross total resection for recurrent glioblastoma. Spatial pattern of recurrence and a review of the literature as a basis for target volume definition. Strahlenther Onkol 193:897–909
Niyazi M, Siefert A, Schwarz SB et al (2011) Therapeutic options for recurrent malignant glioma. Radiother Oncol 98:1–14
Fogh SE, Andrews DW, Glass J et al (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28:3048–3053
Archavlis E, Tselis N, Birn G, Ulrich P, Baltas D, Zamboglou N (2013) Survival analysis of HDR brachytherapy versus reoperation versus temozolomide alone: a retrospective cohort analysis of recurrent glioblastoma multiforme BMJ Open 3(3):e002262. https://doi.org/10.1136/bmjopen-2012-002262
Lawrence YR, Li XA, el Naqa I et al (2010) Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 76:S20–7
Frischer JM, Marosi C, Woehrer A et al (2016) Gamma knife in recurrent glioblastoma. Stereotact Funct Neurosurg 94:265–272
Tselis N, Kolotas C, Birn G et al (2007) CT-guided interstitial HDR brachytherapy for recurrent glioblastoma multiforme. Long-term results. Strahlenther Onkol 183:563–570
Milickovic N, Tselis N, Karagiannis E, Ferentinos K (2017) Iridium-Knife: Another knife in radiation oncology. Brachytherapy 16:884–892
Barbarite E, Sick JT, Berchmans E et al (2016) The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg Rev 40(2):195–211. https://doi.org/10.1007/s10143-016-0727-6
Amelio D, Amichetti M (2012) Radiation therapy for the treatment of recurrent glioblastoma: an overview. Cancers (Basel) 4:257–280
Redmond KJ, Mehta M (2015) Stereotactic radiosurgery for glioblastoma. Cureus 7:e413
Prados MD, Gutin PH, Phillips TL et al (1992) Interstitial brachytherapy for newly diagnosed patients with malignant gliomas: the UCSF experience. Int J Radiat Oncol Biol Phys 24:593–597
Gutin PH, Prados MD, Phillips TL et al (1991) External irradiation followed by an interstitial high activity iodine-125 implant “boost” in the initial treatment of malignant gliomas: NCOG study 6G-82-2. Int J Radiat Oncol Biol Phys 21:601–606
Pedicini P, Florentino A, Simeon V et al (2014) Clinical radiobiology of glioblastoma multiforme. Estimation of tumor control probability from various radiotherapy fractionation schemes. Strahlenther Onkol 190:925–932
Macdonald DR, Cascino TL, Schold SC, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Li J, Wang M, Won M et al (2011) Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys 81:623–630
Muth C, Rubner Y, Semrau S et al (2016) Primary glioblastoma multiforme tumors and recurrence. Strahlenther Onkol 192:146–155
Filippini G, Falcone C, Boiardi A et al (2008) Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro-oncology 10:79–87
Xu J, Fang J, Shen Y, Zhang J, Liu W, Shen H (2011) Should we reoperate for recurrent high-grade astrocytoma? J Neurooncol 105:291–299
Ringel F, Pape H, Sabel M et al (2016) Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro-oncology 18:96–104
Oppenlander ME, Wolf AB, Snyder LA et al (2014) An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 120:846–853
Montemurro N, Perrini P, Blanco MO, Vannozzi R (2016) Second surgery for recurrent glioblastoma: a concise overview of the current literature. Clin Neurol Neurosurg 142:60–64
Franceschi E, Bartolotti M, Tosoni A et al (2015) The effect of re-operation on survival in patients with recurrent glioblastoma. Anticancer Res 35:1743–1748
Nava F, Tramacere I, Fittipaldo A et al (2014) Survival effect of first- and second-line treatments for patients with primary glioblastoma: a cohort study from a prospective registry, 1997–2010. Neuro-oncology 16:719–727
Hervey-Jumper SL, Berger MS (2014) Reoperation for recurrent high-grade glioma: a current perspective of the literature. Neurosurgery 75:491–499
Gorlia T, Stupp R, Brandes AA et al (2012) New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer 48:1176–1184
Seystahl K, Wick W, Weller M (2016) Therapeutic options in recurrent glioblastoma - an update. Crit Rev Oncol Hematol 99:389–408
Weller M, Tabatabai G, Kästner B et al (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res 21:2057–2064
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28:1168–1174
Wick W, Brandes A, Gorlia T et al (2015) LB-05Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro-oncology 17(suppl 5):v1.5–v1. https://doi.org/10.1093/neuonc/nov306
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23:8863–8869
Fokas E, Wacker U, Gross MW, Henzel M, Encheva E, Engenhart-Cabillic R (2009) Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol 185:235–240
Kickingereder P, Hamisch C, Suchorska B et al (2014) Low-dose rate stereotactic iodine-125 brachytherapy for the treatment of inoperable primary and recurrent glioblastoma: single-center experience with 201 cases. J Neurooncol 120:615–623
Fabrini MG, Perrone F, de Franco L et al (2009) Perioperative high-dose-rate brachytherapy in the treatment of recurrent malignant gliomas. Strahlenther Onkol 185:524–529
Mesti T, Ocvirk J (2016) Malignant gliomas: old and new systemic treatment approaches. Radiol Oncol 50:129–138
Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH (2015) Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 11:504–514
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Chatzikonstantinou, N. Zamboglou, E. Archavlis, I. Strouthos, E. Zoga, N. Milickovic, B. Hilaris, D. Baltas, C. Rödel and N. Tselis declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chatzikonstantinou, G., Zamboglou, N., Archavlis, E. et al. CT-guided interstitial HDR-brachytherapy for recurrent glioblastoma multiforme: a 20-year single-institute experience. Strahlenther Onkol 194, 1171–1179 (2018). https://doi.org/10.1007/s00066-018-1358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1358-3
Keywords
- Glioblastoma multiforma
- Recurrent glioma
- Interstitial brachytherapy
- High-dose-rate
- Image-guided radiotherapy