Abstract
Background
For glioblastoma (GBM), multiple prognostic factors have been identified. Semantic imaging features were shown to be predictive for survival prediction. No similar data have been generated for the prediction of progression. The aim of this study was to assess the predictive value of the semantic visually accessable REMBRANDT [repository for molecular brain neoplasia data] images (VASARI) imaging feature set for progression and survival, and the creation of joint prognostic models in combination with clinical and pathological information.
Methods
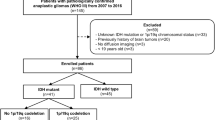
189 patients were retrospectively analyzed. Age, Karnofsky performance status, gender, and MGMT promoter methylation and IDH mutation status were assessed. VASARI features were determined on pre- and postoperative MRIs. Predictive potential was assessed with univariate analyses and Kaplan–Meier survival curves. Following variable selection and resampling, multivariate Cox regression models were created. Predictive performance was tested on patient test sets and compared between groups. The frequency of selection for single variables and variable pairs was determined.
Results
For progression free survival (PFS) and overall survival (OS), univariate significant associations were shown for 9 and 10 VASARI features, respectively. Multivariate models yielded concordance indices significantly different from random for the clinical, imaging, combined, and combined + MGMT models of 0.657, 0.636, 0.694, and 0.716 for OS, and 0.602, 0.604, 0.633, and 0.643 for PFS. “Multilocality,” “deep white-matter invasion,” “satellites,” and “ependymal invasion” were over proportionally selected for multivariate model generation, underlining their importance.
Conclusions
We demonstrated a predictive value of several qualitative imaging features for progression and survival. The performance of prognostic models was increased by combining clinical, pathological, and imaging features.
Zusammenfassung
Einleitung
Für das Glioblastoma multiforme wurden bereits multiple prognostische Faktoren identifiziert. Semantische Bildeigenschaften zeigten eine prognostische Aussagekraft für das Überleben. Die Wertigkeit zur Vorhersage der Krankheitsprogression bleibt unklar. Ziele der Studie waren die prognostische Wertigkeit der sog. VASARI-Bildeigenschaften für Krankheitsprogression und Überleben zu evaluieren und kombinierte Vorhersagemodelle unter Beachtung klinischer und pathologischer Informationen zu generieren.
Methoden
Von 189 Patienten wurden retrospektiv Alter, Karnofsky-Index, Geschlecht, MGMT-Promotor-Methylierung und IDH-Mutationsstatus erfasst. “Visually Accessable REMBRANDT [Repository for Molecular Brain Neoplasia Data] Images”(VASARI)-Eigenschaften wurden in prä- und postoperativen MRT-Studien bestimmt. Die Vorhersagekraft einzelner Eigenschaften wurde univariat und anhand von Kaplan-Meyer-Kurven analysiert. Nach Variablenselektion und Resampling wurden multivariate Cox-Regressionsmodelle generiert. Die prädiktive Vorhersagekraft der Modelle wurde mit Testpatienten ermittelt und die Modelle wurden miteinander verglichen. Die Selektionsfrequenz einzelner Variablen und Variablenpaare wurden analysiert.
Ergebnisse
Mit dem progressionsfreien Überleben (PFS) und dem Gesamtüberleben (OS) waren 9 bzw. 10 VASARI-Eigenschaften in der univariaten Analyse signifikant assoziiert. Die Vorhersagekraft der multivariaten Modelle (klinisch, Bildgebung, kombiniert, kombiniert + MGMT) waren mit Konkordanzindizes von je 0,657, 0,636, 0,694 und 0,716 für OS und 0,602, 0,604, 0,633 und 0,643 für PFS signifikant besser als der Zufall. Die Eigenschaften „Multilokalität“, „Invasion tiefer weißer Hirnstrukturen“, „Satellitenherde“ und „ependymale Invasion“ wurden überproportional für die Generierung der multivariaten Modelle selektiert und unterstreichen deren Bedeutung.
Schlussfolgerung
Wir konnten für die VASARI-Eigenschaften eine prädiktive Vorhersagekraft für PFS und OS nachweisen. Die Vorhersagekraft multivariabler Modelle wurde durch Hinzunahme von klinischen und pathologischen Informationen verbessert.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C et al (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro-Oncology 18:v1–v75. https://doi.org/10.1093/neuonc/now207
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F et al (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Zinn PO, Sathyan P, Mahajan B, Bruyere J, Hegi M, Majumder S et al (2012) A novel volume-age-KPS (VAK) glioblastoma classification identifies a prognostic cognate microRNA-gene signature. PLoS ONE 7:1–9. https://doi.org/10.1371/journal.pone.0041522
Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF (2005) MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 26:2466–2474
Curran WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ et al (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710
Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D et al (2008) Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro-oncology 10:79–87. https://doi.org/10.1215/15228517-2007-038
Burth S, Kickingereder P, Eidel O, Tichy D, Bonekamp D, Weberling L et al (2016) Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro-Oncology 18:1673–1679. https://doi.org/10.1093/neuonc/now122
Putz F, Putz T, Goerig N, Knippen S, Gryc T, Eyüpoglu I et al (2016) Improved survival for elderly married glioblastoma patients. Strahlenther Onkol 192:797–805. https://doi.org/10.1007/s00066-016-1046-0
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‑year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Combs SE, Edler L, Rausch R, Welzel T, Wick W, Debus J (2013) Generation and validation of a prognostic score to predict outcome after re-irradiation of recurrent glioma. Acta Oncol (Madr) 52:147–152. https://doi.org/10.3109/0284186X.2012.692882
Kessel KA, Hesse J, Straube C, Zimmer C, Schmidt-Graf F, Schlegel J et al (2017) Validation of an established prognostic score after re-irradiation of recurrent glioma. Acta Oncol (Madr) 56:1–5. https://doi.org/10.1080/0284186X.2016.1276621
Muth C, Rubner Y, Semrau S, Rühle P‑F, Frey B, Strnad A et al (2016) Primary glioblastoma multiforme tumors and recurrence. Strahlenther Onkol 192:146–155. https://doi.org/10.1007/s00066-015-0926-z
Combs SE, Rieken S, Wick W, Abdollahi A, von Deimling A, Debus J et al (2011) Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: one step forward, and one step back? Radiat Oncol 6:115. https://doi.org/10.1186/1748-717X-6-115
Reifenberger G, Hentschel B, Felsberg J, Schackert G, Simon M, Schnell O et al (2012) Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 131:1342–1350. https://doi.org/10.1002/ijc.27385
Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J et al (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27:5743–5750. https://doi.org/10.1200/JCO.2009.23.0805
Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A et al (2010) Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol 28:3838–3843. https://doi.org/10.1200/JCO.2010.30.0582
Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27:65–73
Diehn M, Nardini C, Wang DS, McGovern S, Jayaraman M, Liang Y et al (2008) Identification of noninvasive imaging surrogates for brain tumor gene-expression modules. Proc Natl Acad Sci USA 105:5213–5218. https://doi.org/10.1073/pnas.0801279105
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–576. https://doi.org/10.1227/01.NEU.0000297118.47076.5E
The Cancer imaging Archive (2014) VASARI research project. https://wiki.cancerimagingarchive.net/display/Public/VASARI+Research+Project. Accessed 29 Dec 2016
Mazurowski MA, Desjardins A, Malof JM (2013) Imaging descriptors improve the predictive power of survival models for glioblastoma patients. Neuro-Oncology 15:1389–1394. https://doi.org/10.1093/neuonc/nos335
Gutman DA, Cooper LAD, Hwang SN, Holder CA, Gao J, Aurora TD et al (2013) MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology 267:560–569. https://doi.org/10.1148/radiol.13120118
Rao A, Rao G, Gutman DA, Flanders AE, Hwang SN, Rubin DL et al (2016) A combinatorial radiographic phenotype may stratify patient survival and be associated with invasion and proliferation characteristics in glioblastoma. J Neurosurg 124:1008–1017. https://doi.org/10.3171/2015.4.JNS142732
Zhou H, Vallières M, Bai HX, Su C, Tang H, Oldridge D et al (2017) MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro-Oncology 19:862–870. https://doi.org/10.1093/neuonc/now256
Gittleman H, Lim D, Kattan MW, Chakravarti A, Gilbert MR, Lassman AB et al (2017) An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro-Oncology 19:669–677. https://doi.org/10.1093/neuonc/now208
Szopa W, Burley TA, Kramer-Marek G, Kaspera W (2017) Diagnostic and therapeutic biomarkers in glioblastoma: current status and future perspectives. Biomed Res Int. https://doi.org/10.1155/2017/8013575
Narang S, Lehrer M, Yang D, Lee J, Rao A (2016) Radiomics in glioblastoma : current status, challenges and potential opportunities. Transl Cancer Res 5:383–397. https://doi.org/10.21037/tcr.2016.06.31
Peeken JC, Nüsslin F, Combs SE (2017) “Radio-oncomics”: the potential of radiomics in radiation oncology. Strahlenther Onkol 193:767–779. https://doi.org/10.1007/s00066-017-1175-0
Kickingereder P, Radbruch A, Burth S, Wick A, Heiland S, Schlemmer H‑P et al (2016) MR perfusion-derived hemodynamic parametric response mapping of bevacizumab efficacy in recurrent glioblastoma. Radiology 279:542–552. https://doi.org/10.1148/radiol.2015151172
Piroth MD, Holy R, Pinkawa M, Stoffels G, Kaiser HJ, Galldiks N et al (2011) Prognostic impact of postoperative, pre-irradiation 18F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol 99:218–224. https://doi.org/10.1016/j.radonc.2011.03.006
Leitzen C, Wilhelm-Buchstab T, Schmeel LC, Garbe S, Greschus S, Müdder T et al (2016) MRI during radiotherapy of glioblastoma. Strahlenther Onkol 192:481–488. https://doi.org/10.1007/s00066-016-0983-y
Dickerson E, Davenport MS, Syed F, Stuve O, Cohen JA, Rinker JR et al (2017) Effect of template reporting of brain MRIs for multiple sclerosis on report thoroughness and neurologist-rated quality: results of a prospective quality improvement project. J Am Coll Radiol 14:371–379.e1. https://doi.org/10.1016/j.jacr.2016.09.037
Grossmann P, Gutman DA, Dunn WD, Holder CA, Aerts HJWL (2016) Imaging-genomics reveals driving pathways of MRI derived volumetric tumor phenotype features in glioblastoma. BMC Cancer 16:611. https://doi.org/10.1186/s12885-016-2659-5
Gutman DA, Dunn WD, Grossmann P, Cooper LAD, Holder CA, Ligon KL et al (2015) Somatic mutations associated with MRI-derived volumetric features in glioblastoma. Neuroradiology 57:1227–1237. https://doi.org/10.1007/s00234-015-1576-7
Rios Velazquez E, Meier R, Dunn WD, Alexander B, Wiest R, Bauer S et al (2015) Fully automatic GBM segmentation in the TCGA-GBM dataset: prognosis and correlation with VASARI features. Sci Rep 5:16822. https://doi.org/10.1038/srep16822
Meier R, Porz N, Knecht U, Loosli T, Schucht P, Beck J et al (2017) Automatic estimation of extent of resection and residual tumor volume for patients with glioblastoma. J Neurosurg. https://doi.org/10.3171/2016.9.JNS16146
Funding
The work was funded in part by Deutsches Konsortium für Translationale Krebsforschung (DKTK), Partner Site Munich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.C. Peeken, J. Hesse, B. Haller, K.A. Kessel, F. Nüsslin, and S.E. Combs declare that they have no competing interests.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version). Informed consent was obtained from all patients included in the study.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Peeken, J.C., Hesse, J., Haller, B. et al. Semantic imaging features predict disease progression and survival in glioblastoma multiforme patients. Strahlenther Onkol 194, 580–590 (2018). https://doi.org/10.1007/s00066-018-1276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1276-4