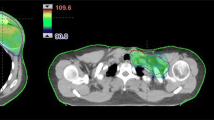
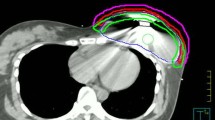
Background and Purpose:
Timing and sequencing of radiotherapy in the context of allogenous breast reconstruction have not been standardized. The aim of the present study was to assess the influence of adjuvant radiotherapy on morbidity and patient satisfaction after allogenous breast reconstruction.
Patients and Methods:
33 patients underwent mastectomy between 1999 and 2008 and had immediate breast reconstruction with an expander placement in subpectoral/epipectoral location. 24 patients had adjuvant chemotherapy. Adjuvant external-beam radiotherapy with a median dose of 50.4 Gy was given after expander filling and on average 5.2 months prior to placement of the definitive implant. 22 patients with the definitive implant were considered for analysis of capsular fibrosis rate. Questionnaires were sent to all patients to assess cosmetic outcome and satisfaction.
Results:
Acute adverse effects were comparable to adjuvant radiotherapy after breast-conserving surgery, resulting in an erythema rate grade 1/2/3 in 21.2%/66.7%/6.1% of patients, respectively. After a mean follow-up of 24.9 months, 9.1%/18.2%/15.2%/9.1% of patients presented a capsular fibrosis grade 1/2/3/4, respectively. Severe deformation/asymmetry of the reconstructed breast was seen in 27.3%/33.3% of patients, respectively. Of the 22 patients with definitive implant, five (22.7%) lost the implant due to painful capsular fibrosis. Of these 22 patients, 50% were very satisfied or satisfied with the reconstruction result. Overall, 81% of patients would request breast reconstruction again.
Conclusion:
Adjuvant radiotherapy with the use of a subtotally filled expander prior to definitive allogenous breast reconstruction is feasible with acceptable morbidity. An interdisciplinary consultation concerning the cosmetic outcome and potential side effects is absolutely necessary.
Hintergrund und Ziel:
Das optimale Timing und die Sequenz einer Strahlentherapie im Kontext einer allogenen Brustrekonstruktion sind unklar. Ziel der vorliegenden Arbeit war die Beurteilung des Einflusses der adjuvanten Radiotherapie auf Morbiditat und Zufriedenheit der Patientinnen nach allogener Brustrekonstruktion.
Patienten und Methodik:
33 Patientinnen erhielten zwischen 1999 und 2008 eine Mastektomie mit subpektoraler/epipektoraler Expanderplatzierung. 24 Patientinnen wurden adjuvant chemotherapiert. Die perkutane Strahlentherapie wurde nach Expanderfullung im Mittel 5,2 Monate vor der Implantateinlage mit median 50,4 Gy durchgefuhrt. 22 Patientinnen mit endgultigem Implantat gingen in die Analyse der Kapselfibroserate ein. An alle Patientinnen wurden Fragebogen zur Beurteilung des kosmetischen Ergebnisses und der Zufriedenheit versandt.
Ergebnisse:
Die Akutnebenwirkungen waren mit denen der adjuvanten Strahlentherapie nach brusterhaltender Therapie vergleichbar. Es zeigte sich ein Erythem Grad 1/2/3 in 21,2%/66,7%/6,1% der Falle. Nach einem mittleren Nachbeobachtungszeitraum von 24,9 Monaten zeigten 9,1%/18,2%/15,2%/9,1% der Patientinnen eine Kapselfibrose Grad 1/2/3/4. Eine hochgradige Verformung/Asymmetrie der rekonstruierten Brust wurde in 27,3%/33,3% der Falle gesehen. Bei funf der 22 Patientinnen (22,7%) mit endgultigem Implantat kam es zum Implantatverlust aufgrund schmerzhafter Kapselfibrosierung. Von diesen 22 Patienten waren 50% sehr zufrieden oder zufrieden mit dem Ergebnis des Wiederaufbaus. Insgesamt 81% der Patientinnen wurden wieder eine Brustrekonstruktion durchfuhren lassen.
Schlussfolgerung:
Eine adjuvante Strahlentherapie ist bei weitgehend gefulltem Expander im Rahmen der allogenen Brustrekonstruktion mit akzeptabler Morbiditat moglich. Eine interdisziplinare Beratung hinsichtlich des zu erwartenden kosmetischen Ergebnisses und moglicher Nebenwirkungen ist jedoch unabdingbar.
Similar content being viewed by others
References
Baloq J, Lucas D, DeSouza C, Crilly R. Helical tomotherapy radiation leakage and shielding considerations. Med Phys 2005;32:710–9.
Bortfeld T, Webb S. Single-arc IMRT? Phys Med Biol 2009;54:9–20.
Dörr W, Herrmann T. Second tumors after oncologic treatment. Strahlenther Onkol 2008;184:67–72.
Fiorino C, Dell’Oca I, Pierelli A, et al. Simultaneous integrated boost (SIB) for nasopharynx cancer with helical tomotherapy. A planning study. Strahlenther Onkol 2007;183:497–505.
Followill DS, Nüsslin F, Orton CG. Point/counterpoint: IMRT should not be administered at photon energies greater than 10 MV. Med Phys 2007;34:1877–9.
Georg D, Julia F, Briot E, et al. Dosimetric comparison of an integrated multileaf-collimator versus a conventional collimator. Phys Med Biol 1997;42:2285–303.
Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83–8.
Horowitz YS. Thermoluminescence and thermoluminescent dosimetry, vols I-III. Boca Raton: CRC Press, 1984.
Huq MS, Das IJ, Steinberg T, et al. A dosimetric comparison of various multileaf collimators. Phys Med Biol 2002;47:N159–70.
Izewska J, Novotny J, Van Dam J, et al. The influence of the IAEA standard holder on dose evaluated from TLD samples. Phys Med Biol 1996;41:465–73.
Klein EE, Maserang B, Wood R, et al. Peripheral doses from pediatric IMRT. Med Phys 2006;33:2525–31.
Kry SF, Salehpour M, Followill DS, et al. Out-of-field photon and neutron dose equivalents from step-and-shoot intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2005;62:1204–16.
Kry SF, Titt U, Pönisch F, et al. A Monte Carlo model for calculating out-of-field dose from a Varian 6 MV beam. Med Phys 2006;33:4405–13.
Ludwig V, Schwab F, Guckenberger M, et al. Comparison of wedge versus segmented techniques in whole breast irradiation. Effect on dose exposure outside the treatment volume. Strahlenther Onkol 2008;184:307–12.
Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys 1993;20:1709–19.
Mansur DB, Klein EE, Maserang BP. Measured peripheral dose in pediatric radiation therapy: a comparison of intensity-modulated and conformal techniques. Radiother Oncol 2007;82:179–84.
Olofsson J, Georg D, Karlsson M. A widely tested model for head scatter influence on photon beam output. Radiother Oncol 2003;67:225–38.
Palm A, Johansson KA. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field; implications for the long-term morbidity of cancer survivors. Acta Oncol 2007;46:462–73.
Ramsey C, Seibert R, Mahan SL, et al. Out-of-field dosimetry measurements for a helical tomotherapy system. J Appl Clin Med Phys 2006;7:1–11.
Reft CS, Runkel-Muller R, Myrianthopoulos L. In vivo and phantom measurements of the secondary photon and neutron doses for prostate patients undergoing 18 MV IMRT. Med Phys 2006;33:3734–42.
Rochet N, Sterzing F, Jensen A, et al. Helical tomotherapy as a new treatment technique for whole abdominal irradiation. Strahlenther Onkol 2008;184:145–9.
Schneider U, Kaser-Hotz B. A simple dose-response relationship for modeling secondary cancer incidence after radiotherapy. Z Med Phys 2005;15:31–7.
Schneider U, Lomax A, Timmermann B. Second cancers in children treated with modern radiotherapy techniques. Radiother Oncol 2008;89:135–40.
Sharma SD, Upreti RR, Laskar S, et al. Estimation of risk of radiation-induced carcinogenesis in adolescents with nasopharyngeal cancer treated using sliding window IMRT. Radiother Oncol 2008;86:177–81.
Sterzing F, Schubert K, Sroka-Perez G, et al. Helical tomotherapy experiences of the first 150 patients in Heidelberg. Strahlenther Onkol 2008;184:8–14.
Van der Giessen PH. Collimator-related radiation dose for different cobalt machines and linear accelerators. Int J Radiat Oncol Biol Phys 1996;35:399–405.
Van der Giessen PH. A simple and generally applicable method to estimate the peripheral dose in radiation teletherapy with high energy x-rays or gamma radiation. Int J Radiat Oncol Biol Phys 1996;35:1059–68.
Van der Giessen PH. Measurement of the peripheral dose for the tangential breast treatment technique with Co-60 gamma radiation and high energy X-rays. Radiother Oncol 1997;42:257–64.
Verellen D, Vanhavere F. Risk assessment of radiation-induced malignancies based on whole-body equivalent dose estimates for IMRT treatment in the head and neck region. Radiother Oncol 1999;53:199–203.
Voigt A. Entwicklung eines Thermolumineszenzdosimetrieverfahrens für Photonen und Neutronen zur Anwendung im Niedrigdosisbereich außerhalb der Bestrahlungsfelder in der klinischen Strahlentherapie. M.Sc. Thesis, Physikalisch-Astronomische Fakultät der Friedrich-Schiller-Universität Jena, 2005.
Voordeckers M, Everaert H, Tournel K, et al. Longitudinal assesment of parotid function in patients receiving tomotherapy for head-and-neck cancer. Strahlenther Onkol 2008;184:400–5.
Webb S. Contemporary IMRT: developing physics and clinical implementation. Bristol and Philadelphia: Institute of Physics Publishing, 2004.
Wiezorek T, Voigt A, Metzger N, et al. Experimental determination of peripheral doses for different IMRT techniques delivered by a Siemens linear accelerator. Strahlenther Onkol 2008;184:73–9.
Xu XG, Bednarz B, Paganetti H. A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction. Phys Med Biol 2008;53:193–241.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piroth, M.D., Piroth, D.M., Pinkawa, M. et al. Immediate Reconstruction with an Expander/Implant Following Ablatio Mammae because of Breast Cancer. Strahlenther Onkol 185, 669–674 (2009). https://doi.org/10.1007/s00066-009-2013-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-009-2013-9