Abstract
Background
The use of extracorporeal membrane oxygenation (ECMO) for patients with acute respiratory distress syndrome (ARDS) has increased substantially. With modern trials supporting its efficacy, ECMO has become an important tool in the management of severe ARDS.
Objectives
The objectives of this paper are to discuss ECMO physiology and configurations used for patients with ARDS, review evidence supporting the use of ECMO for ARDS, and discuss aspects of management during ECMO.
Conclusion
Current evidence supports the use of ECMO, combined with an ultra-lung-protective approach to mechanical ventilation, in patients with ARDS who have refractory hypoxemia or hypercapnia with severe respiratory acidosis. Furthermore, data suggest that center volume and experience are important factors in the care of patients receiving ECMO. The use of extracorporeal technologies in expanded patient populations and the optimal management of patients during ECMO remain areas of investigation.
This article is freely available.
Zusammenfassung
Hintergrund
Der Einsatz der extrakorporalen Membranoxygenierung (ECMO) bei Patienten mit akutem Atemnotsyndrom (ARDS) hat sich wesentlich erhöht. Da ihre Wirksamkeit durch rezente Studien belegt ist, ist die ECMO inzwischen zu einem essenziellen Instrument in der Behandlung des schweren ARDS geworden.
Ziele
Ziel dieser Veröffentlichung ist es, die ECMO-Physiologie und die bei Patienten mit ARDS verwendeten Konfigurationen zu diskutieren, die Evidenz für den Einsatz der ECMO beim ARDS zu überprüfen und Aspekte des Managements während der ECMO zu erörtern.
Schlussfolgerung
Die derzeit verfügbare Evidenz spricht für den Einsatz der ECMO in Kombination mit einem ultra-lungenprotektiven Ansatz für die mechanische Beatmung von ARDS-Patienten, die eine refraktäre Hypoxämie oder Hyperkapnie mit schwerer respiratorischer Azidose aufweisen. Außerdem deuten die Daten darauf hin, dass das Volumen und die Erfahrung des Zentrums relevante Faktoren bei der Versorgung von mit ECMO behandelten Patienten sind. Weiter zu untersuchen sind nach wie vor der Einsatz extrakorporaler Technologien bei erweiterten Patientengruppen und das optimale Management von Patienten während der ECMO.
Dieser Artikel ist frei verfügbar.
Similar content being viewed by others
Introduction
The use of extracorporeal membrane oxygenation (ECMO) for patients with acute respiratory distress syndrome (ARDS) has increased substantially since it was first described in 1972 [16]. Spurred by advancements in extracorporeal technology, experience gained during the influenza A (H1N1) and coronavirus disease 2019 (COVID-19) pandemics, and modern trials supporting its efficacy, ECMO has become an important tool in the management of severe ARDS.
ECMO physiology and configurations
ECMO functions by draining blood from a central vein and pumping it through a gas exchange device after which the blood is reinfused into the patient. The gas exchange device, or membrane lung, facilitates oxygenation of the blood and removal of carbon dioxide (CO2) via diffusion across a semipermeable membrane. Blood flows along one side of the membrane, and sweep gas, a mixture of air and oxygen in proportions set by a blender, flows along the other side. Oxygen diffuses into the blood and CO2 out of it, after which the well-oxygenated blood is returned to the patient through the ECMO circuit and CO2 gas is released to the environment. Therefore, ECMO can be used to support patients with both refractory hypoxemia and respiratory acidosis [7].
Similar to ECMO, extracorporeal carbon dioxide removal (ECCO2R) is a form of extracorporeal life support that can be utilized when CO2 removal (but not oxygenation) is desired, such as in patients with less severe ARDS, when the application of tidal volumes and respiratory rates (RR) lower than the current standard of care are limited by respiratory acidosis. Because CO2 diffuses efficiently across the membrane lung, ECCO2R may be achieved with lower blood flow rates and smaller ECMO cannulae than those typically required for ECMO. Though appealing in concept, use of ECCO2R for ARDS remains experimental and an area of ongoing investigation [7].
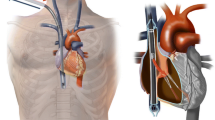
Patients with ARDS are typically supported with venovenous ECMO (VV-ECMO), in which blood is both drained from and reinfused into a central vein. Typically, a drainage cannula is placed in the right or left femoral vein and blood is reinfused via the right or left internal jugular vein (Fig. 1); however femoral venous reinfusion is also possible. Single-site VV-ECMO can be facilitated via use of a bicaval dual-lumen cannula, most frequently inserted via the right internal jugular vein, but proper placement typically requires fluoroscopic imaging guidance and use of echocardiography (Fig. 2). As VV-ECMO operates in series with native circulation, adequate native cardiac output is required to ensure systemic oxygen delivery. In contrast to VV-ECMO, venoarterial configurations utilize venous drainage but reinfuse oxygenated blood into the arterial system, thereby providing hemodynamic support in addition to gas exchange. In patients with ARDS, venoarterial ECMO may be considered when respiratory failure is accompanied by cardiogenic shock [11].
Two-site venovenous extracorporeal membrane oxygenation cannulation. The venous drainage cannula is typically placed in the femoral vein and extends into the inferior vena cava; the venous return cannula is typically placed in the internal jugular vein and extends to the right atrium. Venous blood is drawn from the femoral vein and propelled by the pump into the membrane lung before being returned via the internal jugular vein. (From [30], reprinted with permission of Wolters Kluwer Health, Inc., all rights reserved)
Single-site venovenous extracorporeal membrane oxygenation (ECMO) cannulation. A dual-lumen cannula is typically positioned in the internal jugular vein and terminates in the inferior vena cava. Venous blood from the drainage lumen is drawn into the ECMO circuit from ports positioned in the superior and inferior vena cavae. Oxygenated blood is returned to the second lumen of the same cannula through a port in the right atrium, with blood flow directed across the tricuspid valve. (From [30], reprinted with permission of Wolters Kluwer Health, Inc., all rights reserved)
Evidence for ECMO for respiratory failure and ARDS
Several randomized clinical trials inform our understanding of the appropriate use of ECMO in patients with ARDS. Published in 2009 during the influenza A (H1N1) pandemic, the Conventional Ventilation or ECMO for Severe Acute Respiratory Failure (CESAR) trial randomized patients with severe hypoxemic respiratory failure to referral to an ECMO-capable center versus usual care. CESAR demonstrated improved 6‑month survival without disability in patients referred to an ECMO-capable center (relative risk 0.69, 95% confidence interval [CI] 0.05–0.97; p = 0.03). The CESAR trial emphasizes the benefit associated with ECMO center referral, although the trial design (including the fact that only 76% of patients referred for ECMO ultimately received ECMO, and the lack of a standardized ventilator approach in the control group) limits the conclusions that can be made regarding the effect of ECMO itself [22].
The CESAR trial laid the groundwork for the largest randomized trial of ECMO for severe ARDS, the Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial. In EOLIA, patients were randomized to ECMO combined with an ultra-lung-protective ventilation strategy versus optimal conventional care with a protocolized, lung-protective approach to mechanical ventilation. The trial was terminated early based on prespecified criteria, though still demonstrated that patients who received ECMO had a clinically important reduction in mortality at 60 days in the intention-to-treat analysis (35% versus 46%, relative risk 0.76 [95% CI 0.55–1.04]; p = 0.09) [9].
The EOLIA trial defined inclusion criteria for ECMO that have since become widely used when considering the application of ECMO for patients with ARDS (Table 1). These criteria include refractory hypoxemia (partial pressure of arterial oxygen to fraction of inspired oxygen [PaO2:FiO2] < 50 mm Hg for > 3 h or PaO2:FiO2 < 80 mm Hg for > 6 h despite FiO2 ≥ 0.8 and positive end-expiratory pressure [PEEP] ≥ 10 cm of H2O) and hypercapnia with respiratory acidosis (arterial blood pH < 7.25 with partial pressure of CO2 ≥ 60 mm Hg for > 6 h with ventilator settings targeting plateau pressure ≤ 32 cm of H2O and respiratory rate [RR] increased to 35 breaths per minute) [9].
Several analyses have re-examined the impact of the CESAR and EOLIA trials. An individual patient data meta-analysis that pooled data from both trials showed mortality benefit from ECMO at 90-days (relative risk 0.75 [95% CI 0.6–0.94]; p = 0.013) [10]. A post hoc Bayesian analysis of the EOLIA trial demonstrated high probability of mortality benefit from ECMO, [13] and a systematic review and meta-analysis similarly found mortality benefit from ECMO at 60 days (relative risk 0.73 [95% CI 0.58–0.92]; p = 0.008) [20]. In sum, the data support the use of ECMO for patients with severe ARDS when performed at experienced ECMO centers.
ECMO received considerable attention during the COVID-19 pandemic, as its increased use provided further insights into its efficacy for patients with ARDS. Initial reports found that patients with COVID-19-related ARDS supported with ECMO had survival rates comparable to those observed in patients receiving ECMO for ARDS prior to the pandemic [5]. However, in later phases of the pandemic, the role of ECMO in this specific population was called into question because of a trend toward worsening mortality [6]. Despite these observations, an emulated target trial and a registry-based comparative effectiveness study observed a significant reduction in mortality in patients receiving ECMO for COVID-19-related ARDS with refractory hypoxemia compared to conventional mechanical ventilation [15, 29].
ECMO and ultra-lung-protective ventilation
A growing body of evidence suggests that the clinical benefit of ECMO for ARDS may not only be its ability to correct refractory hypoxemia or respiratory acidosis, but also its ability to facilitate ultra-lung-protective ventilation and thus reduce ventilator-induced lung injury (VILI), a key driver of morbidity and mortality in patients with ARDS [28]. Studies have observed reductions in VILI when tidal volumes are reduced to levels below the standard-of-care (i.e. less than 6 mL/kg of ideal body weight), but in the absence of ECMO, these lower tidal volumes are often complicated by severe hypercapnia and respiratory acidosis [24].
Several studies have demonstrated the ability of ECMO to safely facilitate ultra-lung-protective ventilation. A 2015 systematic review found that average tidal volumes were decreased by a median of 2.4 mL/kg following initiation of ECMO [17]. The EOLIA trial observed reductions in average tidal volume from 6.0 to 3.4 mL/kg and plateau pressure from 29.8 to 24.4 cm H2O after initiation of ECMO [9]. A prospective observational study found that RR, plateau pressure, driving pressure, and mechanical power were all able to be reduced following initiation of ECMO, and that lower driving pressure was associated with improved outcomes [27]. Importantly, other studies have shown that the reductions in mechanical ventilation parameters during ECMO are accompanied by reductions in biomarkers and cytokines associated with VILI, [25] thus supporting a mechanistic link between ultra-lung-protective ventilation and minimization of biotrauma and VILI.
Extracorporeal life support for less severe forms of ARDS
In contrast to ECMO, the use of ECCO2R for less severe forms of ARDS has not yet shown clinical benefit. The phase two Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS (SUPERNOVA) trial demonstrated the feasibility of ECCO2R in controlling respiratory acidosis and facilitating ultra-lung-protective ventilation in patients with moderate ARDS, [8] but a subsequent randomized trial (REST) found no difference in mortality between patients with acute hypoxemic respiratory failure receiving ECCO2R combined with an ultra-lung-protective ventilation strategy versus standard lung-protective ventilation alone [19]. Additional investigation is needed before ECCO2R should be considered clinically for patients with ARDS.
Management of the patient with ARDS during ECMO
As the use of ECMO for ARDS has become more widespread, investigators have studied key aspects of patient management during ECMO, including mechanical ventilation practices, prone positioning, anticoagulation strategies and transfusions.
Data guiding optimal mechanical ventilation parameters during ECMO is limited, but the strategy employed during the EOLIA trial is considered a reasonable standard approach [2]. During EOLIA, ECMO-supported patients were ventilated with a strategy of plateau pressure ≤ 24 cm H2O, PEEP ≥ 10 cm H2O (resulting in driving pressure ≤ 14 cm H2O), FiO2 between 0.3 and 0.5, and RR between 10 and 30 breaths per minute. Gas exchange targets included PaO2 of 65 to 90 mm Hg and PaCO2 ≤ 45 mm Hg [9]. A subsequent international, multicenter prospective cohort study of patients undergoing ECMO for ARDS observed close adherence to the ventilation strategy utilized in the EOLIA trial, thus demonstrating feasibility of achieving these targets. Parameters reported in the cohort included median tidal volume 3.7 ml/kg, median plateau pressure 24 cm H2O, median driving pressure 14 cm H2O, and median RR 14 breaths/min [27].
The use of prone positioning during ECMO is an ongoing area of interest. Several small studies suggested benefit when prone positioning was used in patients with ARDS receiving ECMO, and a systematic review and meta-analysis found a mortality benefit at 28 days [21]. However, a recent randomized controlled trial (PRONECMO) found no difference in successful weaning from ECMO at 60 days (risk difference 0.1% [95% CI −14.9 to 15.2%]), intensive care unit length of stay or 90-day mortality in patients receiving ECMO who were proned versus not proned [26]. Of note, 94% of participants in the trial had COVID-19-related ARDS, and the results thus may not be generalizable to patients with other etiologies of ARDS. While the benefit of prone positioning during ECMO requires further investigation, evidence from PRONECMO suggests that prone positioning may offer only marginal reductions in VILI when patients are already receiving an ultra-lung-protective ventilation strategy.
Patients receiving ECMO are typically managed with continuous anticoagulation because of the risks of circuit-related thrombosis. While the optimal approach to anticoagulation is unknown, favorable outcomes have been demonstrated using a low-dose anticoagulation strategy [3]. Bleeding is a common occurrence during ECMO; thus patients often require transfusion of packed red blood cells during ECMO support. The multicenter PROTECMO study found that red blood cell transfusion was associated with a lower risk of death only when transfused for hemoglobin less than 7 g/dL, similar to the threshold commonly used for other critically ill patients [18].
The Extracorporeal Life Support Organization maintains the largest registry of ECMO-related complications, and in a recent analysis the most frequent complications were bleeding and circuit-related events, such as thrombosis [12]. While eligibility for ECMO may at times be complicated and fraught with complex practical and ethical considerations, most experts maintain that the only absolute contraindication to ECMO for ARDS is irreversible, end-stage lung disease in the absence of candidacy for lung transplantation [7].
ECMO for ARDS: from rescue tool to standard of care?
When evaluating a patient’s candidacy for ECMO, an assessment of the appropriate utilization of this complex technology is warranted. While lung-protective ventilation and prone-positioning remain cornerstones of therapy for ARDS, ECMO should also be included as part of the management algorithm for selected patients with severe ARDS [1]. In a recent systematic review and network meta-analysis that compared and ranked different therapeutic strategies in patients with moderate to severe ARDS, prone positioning and VV-ECMO combined with lung-protective ventilation were associated with a lower 28-day mortality than lung-protective ventilation alone [4]. Furthermore, of the different interventions considered (which also included neuromuscular blockade, inhaled nitric oxide, recruitment maneuvers or high PEEP strategies, and high frequency oscillatory ventilation), prone positioning and VV-ECMO had the highest-ranking probabilities of reducing mortality, although there was no significant difference between the two [4]. When viewed in the context of CESAR, EOLIA, and subsequent and post hoc analyses, the totality of the evidence supports the use of ECMO for patients with severe ARDS [9, 10, 13, 20, 22].
Recently published guidelines also reflect the growing recognition of the role that ECMO plays in the management of ARDS. The 2023 European Society of Intensive Care Medicine (ESICM) guidelines on ARDS strongly recommended (with a moderate level of evidence in favor) that patients with severe ARDS (as defined by the EOLIA trial eligibility criteria) not due to COVID-19 should be managed with ECMO in an ECMO center which meets defined organization standards. This recommendation was also applied to patients with COVID-19-related ARDS, but with a low level of evidence [14]. Similarly, the 2023 Clinical Practice Guideline on ARDS from the American Thoracic Society (ATS) made a conditional recommendation for VV-ECMO in select patients with ARDS, with low certainty of evidence. Additional recommendations and considerations articulated by the ATS guideline include a trial of established ARDS management strategies (e.g. lung-protective ventilation and prone positioning) prior to initiation of ECMO, and emphasis on referral to experienced ECMO centers [23].
Conclusion
Since the first successful application of ECMO for ARDS over 50 years ago, ECMO has become a key tool in the management of patients with severe ARDS. Current evidence supports the use of ECMO, combined with an ultra-lung-protective ventilation strategy, in patients with ARDS who have refractory hypoxemia or hypercapnia with severe respiratory acidosis (as outlined by EOLIA criteria), despite optimal conventional management [1, 9]. Furthermore, the available data and guidelines suggest that center volume and experience are important factors in the care of patients receiving ECMO [23]. The use of extracorporeal technologies in expanded patient populations (such as the use of ECCO2R in less severe ARDS) and the optimal management of patients during ECMO remain ongoing areas of investigation.
References
Abrams D, Ferguson ND, Brochard L et al (2019) ECMO for ARDS: from salvage to standard of care? Lancet Respir Med 7(2):108–110
Abrams D, Schmidt M, Pham T et al (2020) Mechanical ventilation for acute respiratory distress syndrome during extracorporeal life support. Research and practice. Am J Respir Crit Care Med 201(5):514–525
Agerstrand CL, Burkart KM, Abrams DC et al (2015) Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 9(2):590–595
Aoyama H, Uchida K, Aoyama K et al (2019) Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open 2(7):e198116
Barbaro RP, MacLaren G, Boonstra PS et al (2021) Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 398(10307):1230–1238
Barbaro RP, MacLaren G, Boonstra PS et al (2020) Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 1025(7):1071–1078
Brodie D, Slutsky AS, Combes A (2019) Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA 322(6):557–568
Combes A, Fanelli V, Pham T et al (2019) Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med 45(5):592–600
Combes A, Hajage D, Capellier G et al (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378(21):1965–1975
Combes A, Peek GJ, Hajage D et al (2020) ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med 46(11):2048–2057
Conrad SA, Broman LM, Taccone FS et al (2018) The extracorporeal life support organization maastricht treaty for nomenclature in extracorporeal life support. A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med 198(4):447–451
www.elso.org/registry.aspx. Accessed 17 Oct 2023
Goligher EC, Tomlinson G, Hajage D et al (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA 320(21):2251–2259
Grasselli G, Calfee CS, Camporota L et al (2023) ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 49(7):727–759
Hajage D, Combes A, Guervilly C et al (2022) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: an emulated target trial analysis. Am J Respir Crit Care Med 206(3):281–294
Hill JD, O’Brien TG, Murray JJ et al (1972) Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 286(12):629–634
Marhong JD, Munshi L, Detsky M et al (2015) Mechanical ventilation during extracorporeal life support (ECLS): a systematic review. Intensive Care Med 41(6):994–1003
Martucci G, Schmidt M, Agerstrand C et al (2023) Transfusion practice in patients receiving VV ECMO (PROTECMO): a prospective, multicentre, observational study. Lancet Respir Med 11(3):245–255
McNamee JJ, Gillies MA, Barrett NA et al (2021) Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical. JAMA 326(11):1013–1023
Munshi L, Walkey A, Goligher E et al (2019) Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 7(2):163–172
Papazian L, Schmidt M, Hajage D et al (2022) Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med 48(3):270–280
Peek GJ, Mugford M, Tiruvoipati R et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374(9698):1351–1363
Qadir N, Sahetya S, Munshi L et al (2023) An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society Clinical Practice guideline. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202311-2011ST
Richard JC, Marque S, Gros A et al (2019) Feasibility and safety of ultra-low tidal volume ventilation without extracorporeal circulation in moderately severe and severe ARDS patients. Intensive Care Med 45(11):1590–1598
Rozencwajg S, Guihot A, Franchineau G et al (2019) Ultra-protective ventilation reduces biotrauma in patients on venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Crit Care Med 47(11):1505–1512
Schmidt M, Hajage D, Lebreton G et al (2023) Prone positioning during extracorporeal membrane oxygenation in patients with severe ARDS: the PRONECMO randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2023.24491
Schmidt M, Pham T, Arcadipane A et al (2019) Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am J Respir Crit Care Med 200(8):1002–1012
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369(22):2126–2136
Urner M, Barnett AG, Bassi GL et al (2022) Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. BMJ 377:e68723
Agerstrand CL, Bacchetta MD, Brodie D (2014) ECMO for Adult Respiratory Failure: Current Use and Evolving Applications. ASAIO J 60(3):255–262. https://doi.org/10.1097/MAT.0000000000000062
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Abrams and C. Agerstrand write for UpToDate. R. Greendyk, R. Kanade, M. Parekh and P. Lemaitre declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
The supplement containing this article is not sponsored by industry.
Additional information
Redaktion
Christian Karagiannidis, Köln
Stefan Kluge, Hamburg
Thomas Staudinger, Wien
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Scan QR code & read article online
Rights and permissions
About this article
Cite this article
Greendyk, R., Kanade, R., Parekh, M. et al. Respiratory extracorporeal membrane oxygenation. Med Klin Intensivmed Notfmed (2024). https://doi.org/10.1007/s00063-024-01118-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00063-024-01118-y
Keywords
- Acute respiratory distress syndrome
- Extracorporeal life support
- ECMO
- Lung-protective ventilation
- Ventilator-induced lung injury