Abstract
Purpose
To investigate microstructural alterations in white matter in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) using neurite orientation dispersion and density imaging (NODDI) and to assess the potential diagnostic performance of NODDI-derived parameters.
Methods
In this study 14 MCI patients, 14 AD patients, and 14 healthy controls (HC) were recruited. The diffusion tensor imaging(DTI)-derived fractional anisotropy (FA) and NODDI-derived neurite density index (NDI), orientation dispersion index (ODI), and volume fraction of isotropic water molecules (Viso) were calculated from the diffusion data. The tract-based spatial statistics (TBSS) method was used for statistical analysis with one-way ANOVA. The correlations between the parameter values and mini-mental state examination (MMSE) and Montreal cognitive assessment (MoCA) scores were examined. A receiver operating characteristic (ROC) curve was conducted to assess the diagnostic performance of different parameters.
Results
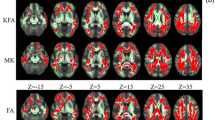
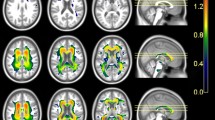
Compared with the HC group, the NDI and ODI values decreased significantly and the Viso values were significantly increased in the MCI and AD groups (p < 0.01, threshold-free cluster enhancement (TFCE)-corrected); however, there were no significant differences in FA values in the MCI group. The NDI, ODI, and Viso values of multiple fibers were significantly correlated with MMSE and MoCA scores. For the diagnosis of AD, the area under the ROC curve (AUC) for the NDI value of the splenium of corpus callosum was larger than the FA value (AUC = 0.885, 0.714, p = 0.042). The AUC of the Viso value of the right cerebral peduncle was larger than FA value (AUC = 0.934, 0.531, p = 0.004).
Conclusion
The NDI is more sensitive to white matter microstructural changes than FA and NODDI could be superior to DTI in the diagnosis of AD.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13.
Radanovic M, Pereira FR, Stella F, Aprahamian I, Ferreira LK, Forlenza OV, Busatto GF. White matter abnormalities associated with Alzheimer’s disease and mild cognitive impairment: a critical review of MRI studies. Expert Rev Neurother. 2013;13:483–93.
Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience. 2014;276:206–15.
Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65.
Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–16.
Merluzzi AP, Dean DC, Adluru N, Suryawanshi GS, Okonkwo OC, Oh JM, Hermann BP, Sager MA, Asthana S, Zhang H, Johnson SC, Alexander AL, Bendlin BB. Age-dependent differences in brain tissue microstructure assessed with neurite orientation dispersion and density imaging. Neurobiol Aging. 2016;43:79–88.
Sepehrband F, Clark KA, Ullmann JF, Kurniawan ND, Leanage G, Reutens DC, Yang Z. Brain tissue compartment density estimated using diffusion-weighted MRI yields tissue parameters consistent with histology. Hum Brain Mapp. 2015;36:3687–702.
Grussu F, Schneider T, Tur C, Yates RL, Tachrount M, Ianuş A, Yiannakas MC, Newcombe J, Zhang H, Alexander DC, DeLuca GC, Gandini Wheeler-Kingshott CAM. Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol. 2017;4:663–79.
Jespersen SN, Bjarkam CR, Nyengaard JR, Chakravarty MM, Hansen B, Vosegaard T, Ostergaard L, Yablonskiy D, Nielsen NC, Vestergaard-Poulsen P. Neurite density from magnetic resonance diffusion measurements at ultrahigh field: comparison with light microscopy and electron microscopy. Neuroimage. 2010;49:205–16.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
Liu Y, Spulber G, Lehtimäki KK, Könönen M, Hallikainen I, Gröhn H, Kivipelto M, Hallikainen M, Vanninen R, Soininen H. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2011;32:1558–71.
Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32:2322.e5–e18.
Wang PN, Chou KH, Chang NJ, Lin KN, Chen WT, Lan GY, Lin CP, Lirng JF. Callosal degeneration topographically correlated with cognitive function in amnestic mild cognitive impairment and Alzheimer’s disease dementia. Hum Brain Mapp. 2014;35:1529–43.
Liu J, Liang P, Yin L, Shu N, Zhao T, Xing Y, Li F, Zhao Z, Li K, Han Y. White matter abnormalities in two different subtypes of amnestic mild cognitive impairment. PLoS ONE. 2017;12:e170185.
Bellucci A, Luccarini I, Scali C, Prosperi C, Giovannini MG, Pepeu G, Casamenti F. Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neurobiol Dis. 2006;23:260–72.
Sun SW, Liang HF, Mei J, Xu D, Shi WX. In vivo diffusion tensor imaging of amyloid-beta-induced white matter damage in mice. J Alzheimers Dis. 2014;38:93–101.
Slattery CF, Zhang J, Paterson RW, Foulkes AJM, Carton A, Macpherson K, Mancini L, Thomas DL, Modat M, Toussaint N, Cash DM, Thornton JS, Henley SMD, Crutch SJ, Alexander DC, Ourselin S, Fox NC, Zhang H, Schott JM. ApoE influences regional white-matter axonal density loss in Alzheimer’s disease. Neurobiol Aging. 2017;57:8–17.
Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer’s disease: findings from neuropathological and neuroimaging studies. J Alzheimers Dis. 2014;42 Suppl 4:S421-9.
Calderon-Garcidueñas AL, Duyckaerts C. Alzheimer disease. Handb Clin Neurol. 2017;145:325–37.
Colgan N, Siow B, O’Callaghan JM, Harrison IF, Wells JA, Holmes HE, Ismail O, Richardson S, Alexander DC, Collins EC, Fisher EM, Johnson R, Schwarz AJ, Ahmed Z, O’Neill MJ, Murray TK, Zhang H, Lythgoe MF. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer’s disease. Neuroimage. 2016;125:739–44.
Merluzzi AP, Dean DC 3rd, Adluru N, Suryawanshi GS, Okonkwo OC, Oh JM, Hermann BP, Sager MA, Asthana S, Zhang H, Johnson SC, Alexander AL, Bendlin BB. Age-dependent differences in brain tissue microstructure assessed with neurite orientation dispersion and density imaging. Neurobiol Aging. 2016;43:79–88.
Li J, Pan P, Huang R, Shang H. A meta-analysis of voxel-based morphometry studies of white matter volume alterations in Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36:757–63.
Pettigrew C, Soldan A, Sloane K, Cai Q, Wang J, Wang MC, Moghekar A, Miller MI, Albert M; BIOCARD Research Team. Progressive medial temporal lobe atrophy during preclinical Alzheimer’s disease. Neuroimage Clin. 2017;16:439–46.
Mormino EC, Papp KV. Amyloid accumulation and cognitive decline in clinically normal older individuals: implications for aging and early alzheimer’s disease. J Alzheimers Dis. 2018;64(s1):S633–46.
Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, Brodtmann A, Rowe CC, Villemagne VL, Connelly A. Reply: Cortical tau pathology: a major player in fibre-specific white matter reductions in Alzheimer’s disease? Brain. 2018;141:e45.
Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;24:547–57.
Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84.
Acknowledgements
This work was supported by the Natural Scientific Foundation of China (Grant number 30870713, PI: Hongyan Ni) and the Tianjin Natural Science Foundation Project (Grant number 16JCYBJC25900, PI: Hongyan Ni). The authors would like to thank Yuanyuan Chen from the Tianjin International Joint Research Center for Neural Engineering, the Academy of Medical Engineering and Translational Medicine, Tianjin University for assistance in data processing and thank Chunchao Ma from the Department of Neurology, Tianjin First Central Hospital for assistance in providing patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
X. Fu, S. Shrestha, M. Sun, Q. Wu, Y. Luo, X. Zhang, J. Yin and H. Ni declare that they have no competing interests.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Fu, X., Shrestha, S., Sun, M. et al. Microstructural White Matter Alterations in Mild Cognitive Impairment and Alzheimer’s Disease. Clin Neuroradiol 30, 569–579 (2020). https://doi.org/10.1007/s00062-019-00805-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-019-00805-0