Abstract
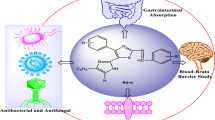
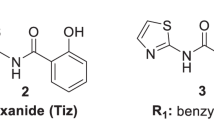
The low oral bioavailability of drugs can be principally attributed to their low solubility, low permeability, and low dissolution rate in the gastrointestinal tract. Considering the low lab-to-market success rate, it is highly important to evaluate these bioavailability factors in the early stages of drug discovery and development. In this work, some physicochemical properties of two new Nitazoxanide analogs (Compounds 1 and 2) were evaluated. Both compounds showed limited aqueous solubility in the pH range of the gastrointestinal tract (16 and 18 μg/mL, respectively), and Log P values were 1.01 and 1.44, for Compounds 1 and 2, respectively. These values indicate a slight affinity for lipid components. The compounds are comprised of a thiazole ring with basic character and an amide group with acidic character; as a result they present ampholytic behavior, with pKa values of 6.029 and 9.565 for Compound 1, and 4.945 and 8.127 for Compound 2. The apparent permeability was evaluated using an everted rat intestine and was quantified using a high performance liquid chromatography equipped with a UV–Vis detector. The results showed that permeability was limited for both compounds (1.15 × 10−5 cm/s for Compound 1 and 9.09 × 10−7 cm/s for Compound 2) when compared to furosemide (1.16 × 10−5 cm/s), a low permeability standard control. These new compounds were therefore classified as low permeability and low solubility (Class IV) according to the Biopharmaceutical Classification System.





Similar content being viewed by others
References
Adagu IS, Nolder D, Warhurst DC, Rossignol J-F (2002) In vitro activity of nitazoxanide and related compounds against isolates of giardia intestinalis, entamoeba histolytica and trichomonas vaginalis. J Antimicrob Chemother 49:103–111. doi:10.1093/jac/49.1.103
Agricultura, S. de G.D.R.P. y A. 2001. NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio.
Albert A, Serjeant E (1962) Ionization Constants of Acids and Bases: A Laboratory Manual. Australian National University. Canberra, and University of New South Wales, Sydney
Amidon G, Lennernäs H, Shah V, Crison J (1995) A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12:413–420
Amidon G, Sinko P, Fleisher D (1988) Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res 5:651–654
Anderson VR, Curran MP (2007) Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs 67:1947–1967. doi:10.2165/00003495-200767130-00015
Borgen L, Okerholm R, Morrison D, Lai A (2003) The influence of gender and food on the pharmacokinetics of sodium oxybate oral solution in healthy subjects. J Clin Pharmacol 43:59–65
Cantelli-Forti G, Guerra MC, Barbaro AM, Hrelia P, Biagi GL, Boreat PA (1986) Relationship between lipophilic character and urinary excretion of nitroimidazoles and nitrothiazoles in rats. J Med Chem 29:555–561
Chan-Bacab MJ, Hernández-Núñez E, Navarrete-Vázquez G (2009) Nitazoxanide, tizoxanide and a new analogue [4-nitro-N-(5-nitro-1,3- thiazol-2-yl)benzamide; NTB] inhibit the growth of kinetoplastid parasites (trypanosoma cruzi and leishmania mexicana) in vitro. J Antimicrob Chemother 63:1292–1293. doi:10.1093/jac/dkp117
Chawla S, Ghosh S, Sihorkar V, Nellore R, Kumar TR, Srinivas NR (2006) High-performance liquid chromatography method development and validation for simultaneous determination of five model compounds, antipyrine, metoprolol, ketoprofen, furosemide and phenol red, as a tool for the standardization of rat in situ intestinal per. Biomed Chromatogr 20:349–357
Damião MCFCB, Pasqualoto KFM, Polli MC, Parise-filho R (2014) To be drug or prodrug : structure-property exploratory approach regarding oral bioavailability. J Pharm Pharm Sci 17:532–540
Drug Bank [WWW Document] 2005. http://www.drugbank.ca/drugs/DB00507,consulted October 15th, 2016
European Parliament, European Council 2010. Directive 2010/63/EU 33–79.
FDA-2000- Guidance for Industry; Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release
Fox LM, Saravolatz LD (2005) Nitazoxanide: a new thiazolide antiparasitic agent. Clin Infect Dis 40:1173–1180. doi:10.1086/428839
Gantala V, Ramanathan S, N. K. N, Mansor SM, Sattar MunavvarAbdul, Khan MA, Navaratnamand V (2006) Permeability of atenolol and propranolol in the presence of dimethyl sulfoxide in rat single-pass intestinal perfusion assay with liquid chromatography/UV detection. Biomed Chromatogr 21:484–490
Gilles HM, Hoffman PS (2002) Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends Parasitol 18:95–97. doi:10.1016/S1471-4922(01)02205-X
Hendriksen B, Felix MVS, Bolger MB (2003) The composite solubility versus pH profile and its role in intestinal absorption prediction. AAPS PharmSci 5:E4. doi:10.1208/ps050104
Hernández-Núñez E, Tlahuext H, Moo-Puc R, Torres-Gómez H, Reyes-Martínez R, Cedillo-Rivera R, Nava-Zuazo C, Navarrete-Vazquez G (2009) Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur J Med Chem 44:2975–2984. doi:10.1016/j.ejmech.2009.01.005
Hoffman PS, Sisson G, Croxen Ma, Welch K, Harman WD, Cremades N, Morash MG (2007) Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and campylobacter jejuni. Antimicrob Agents Chemother 51:868–876. doi:10.1128/AAC.01159-06
ICH (2005) Validation of a analytical procedures : text and methodology Q2(R1). Guidance 1994:17
Kashimura J, Nagai Y (2007) Inhibitory effect of palatinose on glucose absorption in everted rat gut. J Nutr Sci Vitaminol 53:87–89. doi:10.3177/jnsv.53.87
Krimsky M, Dagan a, Aptekar L, Ligumsky M, Yedgar S (2000) Assessment of intestinal permeability in rats by permeation of inulin-fluorescein. J Basic Clin Physiol Pharmacol 11:143–153. doi:10.1515/JBCPP.2000.11.2.143
Kuhlmann J (2000) Responsibilities of clinical pharmacology in the early phase of drug development. Med Klin 95:31–40. (1 Spec)
Lennernäs H (2007) Modeling gastrointestinal drug absorption requires more in vivo biopharmaceutical data: experience from in vivo dissolution and permeability studies in humans. Curr Drug Metab 8:645–57
Lv C, C. W, Wang X, Yao H, Li R, Wang B, Guo R (2014) The influence of food on the Pharmacokinetics of amlodipine and losartan after single-DOSE of its compound tablets in healthy chinese subjects. Drug Res 64:229–235. doi:10.1055/s-0033-1357143
Metzger JV, AD-MARSEILLW, U.O., FRANCE (1979) Thiazole and its derivatives. The Chemistry of Heterocyclic Compounds. Wiley, Marsella France, p 45
Müller J, Wastling J, Sanderson S, Müller N, Hemphill A (2007) A novel Giardia lamblia nitroreductase, G1NR1, interacts with nitazoxanide and other thiazolides. Antimicrob Agents Chemother 51:1979–1986. doi:10.1128/AAC.01548-06
Secretaría de Salud; NORMA Oficial Mexicana NOM-177-SSA1-1998, Que establece las pruebas y procedimientos para demostrar que un medicamento es intercambiable. Requisitos a que deben sujetarse los terceros autorizados que realicen las pruebas. México, 1998. 1–43.
Nonoate, P. 2014. Product Information, 178948
Scior T, Lozano-Aponte J, Ajmani S, Hernández-Montero E, Chávez-Silva F, Hernández-Núñez E, Moo-Puc R, Fraguela-Collar A, Navarrete-Vázquez G (2015) Antiprotozoal nitazoxanide derivatives: synthesis, bioassays and QSAR study combined with docking for mechanistic. Insight Curr Comput Aided-Drug Des 11:21–31. doi:10.2174/1573409911666150414145937
Sisson G, Goodwin A, Raudonikiene A, Hughes NJ, Mukhopadhyay AK, Berg DE, Hoffman PS (2002) Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob Agents Chemother 46:2116–2123. doi:10.1128/AAC.46.7.2116-2123.2002
Stachulski AV, Pidathala C, Row EC, Sharma R, Berry NG, Iqbal M, Bentley J, Allman Sa, Edwards G, Helm A, Hellier J, Korba BE, Semple JE, Rossignol J-F (2011) Thiazolides as novel antiviral agents: I. inhibition of hepatitis B virus replication. J Med Chem 54:4119–4132. doi:10.1021/jm200153p.Thiazolides
Stockis A1, Allemon AM, De Bruyn S, Gengler C (2002) Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses. Int J Clin Pharmacol Ther 40:213–20
Valladares-Méndez A, Hernández-Núñez E, Cedillo-Rivera R, Moo-Puc R, Barbosa-Cabrera E, Orozco-Castellanos LM, Rivera-Leyva JC, Navarrete-Vázquez G (2014) Synthesis, in vitro and in vivo giardicidal activity, and pharmacokinetic profile of a new nitazoxanide analog. Med Chem Res 23:3157–3164. doi:10.1007/s00044-013-0893-9
Yaheya, M., Ismail, M. 2009. Review article. DRUG-food interactions and role of pharmacist. Asian J Pharm Clin Res 2(4), October–December 2009
Acknowledgments
Part of this work was carried out at the Universidad of Guanajuato and RGV Analítica Industrial Research Laboratory. The authors acknowledge the support received by both institutions, whether as economic support PROMEP 2011 Program, as well as an internal grant from the Faculty of Pharmacy of Universidad Autónoma del Estado de Morelos, Universidad de Guanajuato and RGV Analítica Industrial Research Laboratory. Likewise, the support of the Dirección General de Apoyo a la Investigación y al Posgrado (DAIP) of Universidad de Guanajuato for the revision of the manuscript in English is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Valladares-Méndez, A., García-Flores, M., Navarrete-Vázquez, G. et al. Physicochemical characterization of two new Nitazoxanide analogs with antiparasitic activity. Med Chem Res 26, 9–18 (2017). https://doi.org/10.1007/s00044-016-1749-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1749-x