Abstract
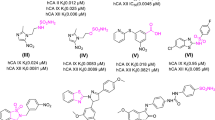
The DNA-dependent protein kinase and phosphoinositide 3-kinase family is one of the most frequently activated enzymes in a wide range of human cancers; consequently, inhibition of DNA-dependent protein kinase and phosphoinositide 3-kinase represents an approach for cancer therapy. In this work, we have designed and synthesized a series of novel 7- or 8-(N-substituted)-2-morpholino quinazolines 3a–f, 5a–e, 7a–e, and 9 from 7- or 8-amino-2-morpholino quinazolin-4-ones (2a, 4a), the 3-methyl analogues (2b, 4b) and the 4-alkyloxy analogues (6a–b, 8). The compounds were subsequently assayed for DNA-dependent protein kinase and phosphoinositide 3-kinase activity. Most compounds were less active than expected in spite of the strong structural resemblance to the previously studied 7- or 8-(O-substituted)-2-morpholino-1,3-benzoxazine inhibitors. Loss of DNA-dependent protein kinase activity for the quinazolin-4-ones (3a–d and 5a–d) has been attributed to tautomerization to the aromatic enol (4-OH) tautomers. Aromatization of the heterocyclic ring could alter the conformation, and thus binding position, resulting in reduced compound-receptor hydrogen bonding of the morpholine oxygen and 4-carbonyl oxygen. The hetero-aromatic compounds 7a–e and 9 also did not show any DNA-dependent protein kinase activity at 10 µM, which supports the above hypothesis. Compound 7c (R=CH2(pyridine-4-yl)) displayed selective phosphoinositide 3-kinase delta activity with 80 % inhibition at 10 µM. Similarly, compounds 5a (8-N-substituted, R=CH2Ph) and 3a (7-N-substituted, R=CH2Ph) showed selective phosphoinositide 3-kinase beta activity with 69 and 61 % inhibition, respectively. Antiplatelet inhibition assays showed that compound 7e with the 4-O-benzyloxy group and 8-CH2(pyridine-3-yl) substituents was found to be the most active (IC50 35 µM).



Similar content being viewed by others
References
Andrs M, Korabecny J, Jun D, Hodny Z, Bartek J, Kuca K (2015) Phosphatidylinositol 3-kinase (PI3K) and phosphatidylinositol 3 kinase related kinase (PIKK) inhibitors: importance of the morpholine ring. J Med Chem 58:41–47
Clapham KM, Rennison T, Jones G, Craven F, Bardos J, Golding BT (2012) Potent enantioselective inhibition of DNA-dependent protein kinase (DNA-PK) by atropisomeric chromenone derivatives. Org Biomol Chem 10:6747–6757
Cushing TD, Metz DP, Whittington DA, McGee LR (2012) PI3Kδ and PI3Kγ as targets for autoimmune and inflammatory diseases. J Med Chem 55:8559–8581
Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJH, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B (2008) Angiogenesis selectively requires the p110α isoform of PI3K to control endothelial cell migration. Nature 453(7195):662–666
Hardcastle IR, Cockcroft X, Curtin NJ, El-Murr MD, Leahy JJJ, Stockley M, Golding BT, Rigoreau L, Richardson C, Smith GCM, Griffin RJ (2005) Discovery of potent chromen-4-one inhibitors of the DNA-dependent protein kinase (DNA-PK) using a small-molecule library approach. J Med Chem 48(24):7829–7846
Heppell J, Al-Rawi J (2014) Functionalisation of quinazolin-4-ones part 1: synthesis of novel 7-substituted-2-thioxo quinazolin-4-ones from 4-substituted-2-aminobenzoic acids and PPh3(SCN)2. J Heterocyclic Chem 51:162–174
Heppell JT, Al-Rawi JMA (2015) Functionalization of quinazolin-4-ones part 2: reactivity of 2-amino-3,4, 5, or 6-nitrobenzoic acids with triphenylphosphine thiocyanate, alkyl isothiocyanates, and further derivatization reactions. J Heterocyclic Chem 52(5):1361–1367
Ihmaid SK, JMA Al-Rawi, Bradley CJ, Angove MJ, Robertson MN (2012) Synthesis, DNA-PK inhibition, anti-platelet activity studies of 2-(N-substituted-3-aminopyridine)-substituted-1,3-benzoxazines and DNA-PK and PI3K inhibition, homology modelling studies of 2-morpholino-(7,8-di and 8-substituted)-1,3-benzoxazines. Eur J Med Chem 57:85–101
Khan I, Ibrar A, Ahmed W, Saeed A (2015) Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: the advances continue. Eur J Med Chem 90:124–169
Lannutti BJ, Meadows SA, Herman SEM, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA (2011) CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of b-cell malignancies, inhibits PI3K signalling and cellular viability. Blood 117:591–594
Leahy JJJ, Golding BT, Griffin RJ, Hardcastle IR, Richardson C, Rigoreau L, Smith GCM (2004) Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg Med Chem Lett 14(24):6083–6087
Liu P, Cheng H, Roberts TM, Zhao JJ (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 8(8):627–644
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298:1912–1934
Marone R, Cmiljanovic V, Giese B, Wymann MP (2008) Targeting phosphoinositide 3-kinase-moving towards therapy. BBA Proteins Proteomics 1784(1):159–185
Morrison R, Belz T, Ihmaid SK, Al-Rawi JMA, Angove MJ (2014) Dual and/or selective DNA-PK, PI3K inhibition and isoform selectivity of some new and known 2-amino-substituted-1,3-benzoxazines and substituted-1,3-naphthoxazines. Med Chem Res 23:4680–4691
Pritchard K, Al-Rawi JMA, Bradley C (2007) Synthesis, identification and anti-platelet evaluation of 2-morpholino substituted benzoxazines. Eur J Med Chem 42(9):1200–1210
Smith GCM, Jackson SP (1999) The DNA-dependent protein kinase. Genes and Dev 13(8):916–934
Workman P, Clarke PA, Raynaud FI, Montfort RLM, Montfor V (2010) Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res 70(6):2146–2157
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
Ethics approval for the use of human blood was obtained from La Trobe University Human Ethics Committee (HEC13-010).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Heppell, J.T., Al-Rawi, J.M.A. Synthesis, structures elucidation, DNA-PK, PI3K and antiplatelet activity of a series of novel 7- or 8-(N-substituted)-2-morpholino-quinazolines. Med Chem Res 25, 1695–1704 (2016). https://doi.org/10.1007/s00044-016-1608-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1608-9