Abstract
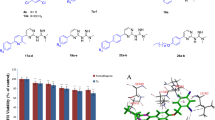
In the present study, we report the synthesis, characterization of new series of thiazolo[3,2-a]pyrimidine-6-carboxylate derivatives 3a–f and 4a–f. The newly synthesized compounds were screened for in vitro antimicrobial and antiviral activities. The probable mode of action of these active compounds was determined through in silico docking study by docking the receptor methionyl-tRNA synthetase and human inosine-5′-monophosphate dehydrogenase (IMPDH) for antibacterial and antiviral activities, respectively. Among the compounds, 4c exhibited excellent in vitro antimicrobial activity against all tested strains with binding and docking energies −35.6 and −12.4 kcal/mol, respectively. The antiviral studies were carried out for the selected compounds in which 4a exhibited 73.69 and 54.42 % of inhibition of buffalopox and camelpox viruses, respectively. Furthermore, compound 4a showed minimum docking and binding energy along with the maximum hydrogen/hydrophobic interaction with IMPDH. The study contributes towards identification and screening of potential antimicrobial and antiviral agent’s against the pathogens.





Similar content being viewed by others
References
Abagyan RA, Totrov MM, Kuznetsov DA (1994) ICM-a new method for protein modelling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15:488–506
Amr AE, Ashraf MM, Salwa FM, Nagla AA, Hammam AG (2006) Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg Med Chem 14:5481–5488
Awadallah FM (2008) Synthesis, pharmacophore modelling, and biological evaluation of novel 5H-thiazolo[3,2-a]pyrimidin-5-one derivatives as 5-HT2A receptor antagonists. Sci Pharm 76:415–438
Balalaie S, Bararjanian M, Rominger F (2006) An efficient one-pot synthesis of 6-aryl-5-cyano-2-thiopyrimidone derivatives and their piperidinium salts, X-ray crystal structures. J Heterocycl Chem 43:821–826
Bhanuprakash V, Hosamani M, Balamurgan V, Gandhale P, Ram ND, Swarup D, Singh RK (2008) In vitro antiviral activity of plant extracts on goatpox virus replication. Indian J Exp Biol 46:120–127
Bhanuprakash V, Prabhu M, Venkatesan G, Balamurugan V, Hosamani M, Pathak KM, Singh RK (2010) Camelpox: epidemiology, diagnosis and control measures. Expert Rev Anti Infect Ther 8:1187–1201
Breman JG, Henderson DA (1998) Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N Engl J Med 339:556–559
Cruickshank R, Duguid JP, Marmion BP, Swain RHA (1975) In: Medical microbiology, 12th edn. Churchil Livingstone, London, p 196–197
Cosier J, Glazer AM (1986) A nitrogen-gas-stream cryostat for general X-ray diffraction studies. J Appl Cryst 19:105–107
Darshan Raj CG, Sarojini BK, Subramanya H, Sreenivasa S, Ravikumar YS, Bhanuprakash V, Yogisharadhya R, Raghavendra R (2012a) In vitro biological activities of new heterocyclic chalcone derivatives. Med Chem Res. doi:10.1007/s00044-012-0193-9
Darshan Raj CG, Sarojini BK, Bhanuprakash V, Yogisharadhya R, Kumarswamy BE, Raghavendra R (2012b) Studies on radioprotective and antiviral activities of some bischalcone derivatives. Med Chem Res 21:2671–2679
De Clercq E, Neyts J (2004) Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev Med Virol 14:289–290
Donald FS, Patricia AM, Dee Nord L, Randall CW, Charles RP, Timothy MR, Ganapathi RR, Roland KR, Roberts AS (1987) Novel pyrazolo[3,4-d]pyrimidine nucleoside analog with broad-spectrum antiviral activity. Antimicrob Agents Chemother 31:1535–1541
Fun HK, Loh WS, Sarojini BK, Umesha K, Narayana B (2011) Methyl(2Z)-2-(2-fluoro-4-methoxybenzylidene)-5-(4-methoxyphenyl)-7-methyl-3-oxo-2,3-dihydro-5H-[1,3] thiazolo[3,2-a]pyrimidine-6-carboxylate. Acta Crystallogr Sect E E67:o1913–o1914
Geist JG, Lauw S, Illarionova V, Illarionova B, Fischer M, Grawert T, Rohdich F, Eisenreich W, Kaiser J, Groll M, Scheurer C, Wittlin S, Alonso-Gomez JL, Schweizer WB, Bacher A (2010) Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from mycobacterium tuberculosis and plasmodium falciparum. ChemMedChem 5:1092–1101
Halgren TA (1996a) Merck Molecular force field: I. Molecular gemetric and vibrational frequecies for MMFF94. J Comput Chem 17:553–586
Halgren TA (1996b) Merck molecular force field:II MMFF94Van der Waals and electrostatic parameter for imtermolecular interaction. J Comput Chem 17:520–552
Halgren TA (1996c) Merck molecular force field:V extension of MMFF94 experimental data, additional computational data, and empirical rules. J Comput Chem 17:616–641
Halgren TA (1999a) MMFF:VI MMFF94s option for energy minimisation studies. J Comput Chem 20:720–729
Halgren TA (1999b) MMFF:VII characterization MMFF94, MMFF94s and oter widely available force fields for conformational energies and for inter-molecular interaction energies and geometries. J Comput Chem 20:730–748
Halgren TA, Nachbar RB (1996) Merck molecular force field: IV conformational energies and geometries for MMFF94. J Comput Chem 17:587–615
Kappe CO (2003) The generation of dihydropyrimidine libraries utilizing Biginelli multi-component chemistry. QSAR Comb Sci 22:630–645
Khan MT, Fuskevag OM, Sylte I (2009) Discovery of potent thermolysin inhibitors using structure based virtual screening and binding assays. J Med Chem 52:48–61
Kim SY, Lee J (2003) 3-D-QSAR study and molecular docking of methionyl-tRNA synthetase inhibitors. Bioorg Med Chem 11(24):5325–5331
Krovat EM, Steindl T, Langer T (2005) Recent advances in docking and scoring. Curr Comput Aided Drug Des 1:93–102
Lagu B, Tian D, Chiu G, Nagarathnam D, Fang J, Shen Q, Forray C, Ransom RW, Chang RS, Vyas KP, Zhang K, Gluchowski C (2000) Synthesis and evaluation of furo[3,4-d]pyrimidinones as selective α1a-adrenergic receptor antagonists. Bioorg Med Chem Lett 10(2):175–178
Liu S, Yang L, Jin Z, Huang E, Wan DCC, Lin H, Hua C (2009) Design, synthesis, and biological evaluation of 7H-thiazolo[3,2-b]-1,2,4-triazin-7-one derivatives as novel acetylcholinesterase inhibitors. ARKIVOC (x):333–348
Nehad AA, Nermien MS, Ashraf MM, Abdulla MM (2007) Synthesis, analgesic, and antiparkinsonian profiles of some pyridine, pyrazoline, and thiopyrimidine derivatives. Monatash Chem 138:715–721
Ram VJ, Vandev Berghe DA, Vlietinck AJ (1984) 5-Cyano-6-aryluracil and 2-aminouracil derivatives as potential chemotherapeutic agents. J Heterocycl Chem 21:1307–1312
Ram VJ, Vandev Berghe DA, Vlietinck AJ (1987) Synthese and activities of novel pyrimidines derived from 5-cyano-6-aryl-2-thiouracil. Liebigs Ann Chem 9:797–801
Ram VJ, Goal A, Nath M, Srivastava P (1994) 5-Cyano-2-thiouracils and their derivatives: a new class of leishmanicides. Bioorg Med Chem Lett 4:2653–2656
Ramalingan C, Kwak YW (2008) Tetrachlorosilane catalyzed multicomponent one-step fusion of biopertinent pyrimidine heterocycles. Tetrahedron 64:5023–5031
Rashad AE, Shamroukh AH, Abdel-Megeid RE, Kandeil AM, Mostafa A, Elshesheny R, Ali MA, Banert K (2010) Synthesis and screening of some novel fused thiophene and thienopyrimidine derivatives for anti-avian influenza virus (H5N1) activity. Eur J Med Chem 11:5251–5257
Sidwell W, Smee DF (2000) In vitro and in vivo assay systems for study of influenza virus inhibitors. Antiviral Res 48:1–16
Warnock DW, Johnson EM, Rogers TR (1998) Multi-centre evaluation of the Etest method for antifungal drug susceptibility testing of Candida spp. and Cryptococcus neoformans. BSAC working party on antifungal chemotherapy. J Antimicrob Chemother 42:321–331
Wichmann J, Adam G, Kolczewski S, Mutel V, Woltering T (1999) Structure-activity relationships of substituted 5H-thiazolo[3,2-a]pyrimidines as group 2 metabotropic glutamate receptor antagonists. Bioorg Med Chem Lett 9:1573–1576
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umesha, K., Sarojini, B.K., Darshan Raj, C.G. et al. In vitro and in silico biological studies of novel thiazolo[3,2-a]pyrimidine-6-carboxylate derivatives. Med Chem Res 23, 168–180 (2014). https://doi.org/10.1007/s00044-013-0606-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0606-4