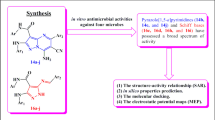
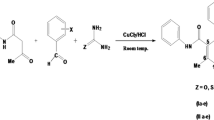
Abstract
In this study, we have amalgamated computational methodologies, viz. Petra, Osiris and Molinspiration (POM) to identify pharmacophores and antipharmacophores for antibacterial/antiviral activities of some 2-pyrazolines derivatives. Lipophilicity and the presence of tautomerism process are the major factors that govern the orientation to antibacterial and/or antiviral activity. On other hand, it was observed that these compounds have two different active sites and can inhibit both antiviral and antibacterial strains. Further, we have carried out receptor-based electrostatic analysis to confirm the electronic, steric and hydrophobic requirements for future modifications. The analyses provide substantial idea about the structural features responsible for their combined antibacterial/antiviral activity and provide guidelines for further modifications, with the aim of improving the activity and selectivity of designed drugs targeting HIV and tuberculosis microorganisms.
Graphical Abstract
The speculative assertions presented in many papers published in many reputed journals are meaningless. Most of the authors discuss of the antiviral activity compared to the antibacterial activity. There authors seem to consider that there is only one tautomeric form taking one mechanism of action for both antiviral and antibacterial activities. This is false; here, we clarify things on the basis of POM analyses.












Similar content being viewed by others
References
Abdel-Wahab BF, Abdel-Aziz HA, Ahmed EM (2009) Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)- 4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur J Med Chem 44:2632–2635
Ahn JH, Kim H-M, Jung SH, Kang SK, Kim KR, Rhee SD, Yang S-D, Cheon HG, Kim SS (2004) Synthesis and DP-IV inhibition of cyano-pyrazoline derivatives as potent anti-diabetic agents. Bioorg Med Chem Lett 14:4461–4465
Ahsan MJ, Samy JG, Dutt KR, Agrawal UK, Yadav BS, Vyas S, Kaur R, Yadav G (2011) Design, synthesis and antimycobacterial evaluation of novel 3-substituted-N-aryl-6,7- dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide analogues. Bioorg Med Chem Lett 21:4451–4453
Ali MA, Shaharyar M, Siddiqui AA (2007a) Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem 42:268–275
Ali MA, Yar MS, Siddiqui AA, Sriram D, Yogeeswari P, De Clercq E (2007b) Synthesis and anti-HIV activity of N′-nicotinoyl–3-(4′-hydroxy-3′-methylphenyl)-5-[substituted phenyl]-2-pyrazolines. Acta Pol Pharm 64:328–423
Ali P, Meshram J, Youssoufi MH, Ben Hadda T (2010) Theoretical calculations and experimental verification of the antibacterial potential of some monocyclic beta-lactames containing two synergetic buried antibacterial pharmacophore sites. Phosphorus Sulfur, Silicon Relat Elem 7:1500–1510
Amir M, Kumar H, Khan SA (2008) Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg Med Chem Lett 18:918–922
Amnerkar ND, Bhusari KP (2010) Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. Eur J Med Chem 45:149–159
Anaflous A, Benchat N, Mimouni M, Abouricha S, Ben Hadda T, El-Bali B, Hakkou A, Hacht B (2004) Armed imidazo [1,2-a]pyrimidines (pyridines): evaluation of antibacterial activity. Lett Drug Des Discov 1:224–229
Banday AH, Mir BP, Lone IH, Suri KA, Sampath Kumar HM (2010) Studies on novel D-ring substituted steroidal pyrazolines as potential anticancer agents. Steroids 75:805–809
Bano S, Javed K, Ahmad S, Rathish IG, Singh S, Alam MS (2011) Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur J Med Chem 46:5763–5768
Bashir R, Ovais S, Yaseen S, Hamid H, Alam MS, Samim M, Singh S, Javed K (2011) Synthesis of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and anti-inflammatory agents. Bioorg Med Chem Lett 21:4301–4305
Ben Hadda T, Benchat N, El-Bali B, Abouricha S, Moueqqit M and Mimouni M (2003) Impact of Dimroth rearrangement on anti-tuberculosis activity of 3-armed-imidazo[1.2-a]pyrimidines IMP (-pyridines) IP. Med Pharm Chem 1–18 (published online, 0301001)
Bennani B, Kerbal A, Daoudi M, Filali Baba B, Al Houari G, Jalbout AF, Mimouni M, Benazza M, Demailly G, Akkurt M, Yýldýrým SÖ, Ben Hadda T (2007) Combined drug design of potential Mycobacterium tuberculosis and HIV-1 inhibitors: 3′,4′-di-substituted-4′H-spiro[isothiochromene-3,5′-isoxazol]-4(1H)-one. Arkivoc xvi:19–40
Chimenti F, Bizzarri B, Manna F, Bolasco A, Secci D, Chimenti P, Granese A, Rivanera D, Lilli D, Scaltrito MM, Brenciaglia MI (2005) Synthesis and in vitro selective anti-Helicobacter pylori activity of pyrazoline derivatives. Bioorg Med Chem Lett 15:603–607
Chimenti F, Fioravanti R, Bolasco A, Manna F, Chimenti P, Secci D, Rossi F, Turini P, Ortuso F, Alcaro S, Cardi MC (2008) Synthesis, molecular modeling studies and selective inhibitory activity against MAO of N1-propanoyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem 43:2262–2267
Chohan ZH, Youssoufi MH, Jarrahpour A, Ben Hadda T (2010a) Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: indolenyl sulfonamide derivatives. Eur J Med Chem 45:1189–1199
Chohan ZH, Sumrra SH, Youssoufi MH, Ben Hadd T (2010b) Metal based biologically active compounds: design, synthesis and antibacterial/antifungal/cytotoxic properties of triazole derived schiff bases and their oxovanadium(IV) complexes. Eur J Med Chem 45:2739–2747
Choudhary MI, Alam MS, Rahman AU, Yousuf S, Wu Y-C, Lin A-S, Shaheen F (2011) Pregnenolone derivatives as potential anticancer agents. Steroids 76:1554–1559
Dadiboyena S, Valente EJ, Hamm AT (2009) A novel synthesis of 1,3,5-trisubstituted pyrazoles through a spiro-pyrazoline intermediate via a tandem 1,3-dipolar cycloaddition/elimination. Tetrahedron Lett 50:291
Dawane BS, Konda SG, Shaikh BM, Chobe SS, Khandare NT, Kamble VT, Bhosale RB (2010) Synthesis and in vitro antimicrobial activity of some new 1-thiazolyl-2-pyrazoline derivatives. Int J Pharm Sci Rev Res 1. www.globalresearchonline.net
Fathi J, Masand V, Jawarkar R, Mouhoub R, Ben Hadda T (2011) POM as efficient tools to predict and improve both antibacterial and antifungal activity of aryl aldazines. J Comput Method Mol Des 1:57–68
Ferreras JA, Gupta A, Amin ND, Basu A, Sinha BN, Worgall S, Jayaprakash V, Quadri LEN (2011) Chemical scaffolds with structural similarities to siderophores of nonribosomal peptide–polyketide origin as novel antimicrobials against Mycobacterium tuberculosis and Yersinia pestis. Bioorg Med Chem Lett 21:6533–6537
Fioravanti R, Bolasco A, Manna F, Rossi F, Orallo F, Ortuso F, Alcaro S, Cirilli R (2010) Synthesis and biological evaluation of N-substituted-3,5-diphenyl-2-pyrazoline derivatives as cyclooxygenase (COX-2) inhibitors. Eur J Med Chem 45:6135–6138
Girisha KS, Kalluraya B, Narayana V, Padmashree P (2011) Synthesis and pharmacological study of 1-acetyl/propyl-3-aryl-5-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-2- pyrazoline. Euro J Med Chem 45:4640–4644
Havrylyuk D, Zimenkovsky B, Vasylenko O, Zaprutko L, Gzella A, Lesyk R (2009) Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur J Med Chem 44:1396–1404
Horna A, Pechova H, Pikuloca A, Churacek J (1986) Chromatography of monomers VI. New derivatives for improved thin-layer chromatographic separation of C1–C18 alkyl esters of acrylic acid. J Chromatogr 367:155–159
Jagrat M, Behera J, Yabanoglu S, Ercan A, Ucar G, Sinha BN, Sankaran V, Basu A, Jayaprakash V (2011) Pyrazoline based MAO inhibitors: synthesis, biological evaluation and SAR studies. Bioorg Med Chem Lett 21:4296–4300
Jarrahpour A, Fathi J, Mimouni M, Ben Hadda T, Sheikh J, Chohan ZH, Parvez A (2011) Petra, Osiris and Molinspiration (POM) together as a successful support in drug design: antibacterial activity and biopharmaceutical characterization of some azo schiff bases. Med Chem Res 19:1–7
Jayaprakash V, Sinha BN, Ucar G, Ercan A (2008) Pyrazoline-based mycobactin analogues as MAO-inhibitors. Bioorg Med Chem Lett 18:6362–6368
Joshi RS, Mandhane PG, Diwakar SD, Dabhade SK, Gill CH (2010) Synthesis, analgesic and anti-inflammatory activities of some novel pyrazolines derivatives. Bioorg Med Chem Lett 20:3721–3725
Jun MA, Park WS, Kang SK, Kim KY, Kim KR, Rhee SD, Bae MA, Kang NS, Sohn S-K, Kim SG, Lee JO, Lee DH, Cheon HG, Kim SS, Ah JH (2008) Synthesis and biological evaluation of pyrazoline analogues with β-amino acyl group as dipeptidyl peptidase IV inhibitors. Eur J Med Chem 43:1889–1902
Karuppasamy M, Mahapatra M, Yabanoglu S, Ucar G, Sinha BN, Basu A, Mishra N, Sharon A, Kulandaivelu U, Jayaprakash V (2011) Development of selective and reversible pyrazoline based MAO-A inhibitors: synthesis, biological evaluation and docking studies. Bioorg Med Chem 18:1875–1881
Kuzmenok NM, Koval’chuk TA, Zvonok AM (2008) Recyclization of 5-hydroxy-2- pyrazolines in the reaction with N-nucleophiles. Arkivocv ix:126–132
Lee M, Brockway O, Dandavati A, Tzou S, Sjoholm R, Satam V, Westbrook C, Mooberry SL, Zeller M, Babu B, Lee M (2011) A novel class of trans-methylpyrazoline analogs of combretastatins: synthesis and in vitro biological testing. Eur J Med Chem 46:3099–3104
Ly P-C, Li D–D, Li Q-S, Lu X, Xiao Z-P, Zhu H-L (2011) Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives as EGFR TK inhibitors and potential anticancer agents. Bioorg Med Chem Lett 21:5374–5377
Manna K, Agrawal YK (2010) Design, synthesis, and antitubercular benzofuran-5-aryl-1-pyrazolylcarbonyl-4-oxo-naphthyridin analogs. Eur J Med Chem 45:3831–3839
Matysiak J, Niewiadomy A (2003) Synthesis and antimycotic activity of N-azolyl-2,4-dihydroxythiobenzamides. Bioorg Med Chem 11:2285–2291
Mishra N, Sasmal D (2011) Development of selective and reversible pyrazoline based MAO-B inhibitors: virtual screening, synthesis and biological evaluation. Bioorg Med Chem Lett 21:1969–1973
Parekh S, Bhavsar D, Savant M, Thakrar S, Bavishi A, Parmar M, Vala H, Radadiya A, Pandya N, Serly J, Molnár J, Shah A (2011) Synthesis of some novel benzofuran-2-yl(4,5-dihyro-3,5-substituted diphenylpyrazol-1-yl) methanones and studies on the antiproliferative effects and reversal of multidrug resistance of human MDR1-gene transfected mouse lymphoma cells in vitro. Eur J Med Chem 46:1942–1948
Parvez A, Jyotsna M, Youssoufi MH, Ben Hadda T (2010) Bioinformatic prediction and experimental verification of antibacterial potential of some monocyclic β-lactams containing two synergetic buried antibacterial pharmacophore sites (part II). Phosphorus Sulfur, Silicon Relat Elem 185:1500–1510
Parvez A, Meshram JS and Ben Hadda T (2011) POM validation and structure based drug design: a novel transpeptidase inhibitor with least bacterial resistance. In: International conference on chemistry for mankind: innovative ideas in life sciences, ICCM-2011, Nagpur, India, 09–11 February. ICCM-2011. http://www.iccm2011-rtmnu.org
Rajendra PY, Lakshmana RA, Prasoona K, Murali K, Ravi KP (2005) Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-prazolines and 3-(2″-hydroxynaphthalen-1″-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 15:5030
Rani M, Yousuf M (2011) Synthesis, studies and in vitro antibacterial activity of some 5-(thiophene-2-yl)-phenyl pyrazoline derivatives. J Saudi Chem Soc. doi:10.1016/j.jscs.2011.09.002
Rani M, Yusuf M, Khan SA (2011) Synthesis and in vitro antibacterial activity of [5-(furan-2-yl)-phenyl]-4,5-carbothioamide-pyrazolines. J Saudi Chem Soc. doi:10.1016/j.jscs.2011.02.012
Rani M, Yusuf M, Khan SA, Sahota PP, Pandove G (2011) Synthesis, studies and in-vitro antibacterial activity of N-substituted 5-(furan-2-yl)-phenyl pyrazolines. Arabian J Chem. doi:10.1016/j.arabjc.2010.10.036
Rathish IG, Javed K, Ahmad S, Bano S, Alam MS, Pillai KK, Singh S, Bagchi V (2009) Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg Med Chem Lett 19:255–258
Rispens MT, Keller E, Lange BD, Zijlstra RWJ, Feringa BL (1994) Asymmetric 1,3-dipolar cycloadditions to 5-(R)-menthyloxy-2(5H)-furanone. Tetrahedron Asymm 5:607–624
Sahoo A, Yabanoglu S, Sinha BN, Ucar G, Basu A, Jayaprakash V (2010) Towards development of selective and reversible pyrazoline based MAO-inhibitors: synthesis, biological evaluation and docking studies. Bioorg Med Chem Lett 20:132–136
Sanderfer PO (1965) Decomposition of 2-pyrazolines. Annual report, University of Florida, August
Shaharyar M, Siddiqui AA, Ali MA, Sriram D, Yogeeswari P (2006) Synthesis and in vitro antimycobacterial activity of N1-nicotinoyl-3-(4′-hydroxy-3′-methyl phenyl)-5-[(sub)phenyl]-2-pyrazolines. Bioorg Med Chem Lett 16:3947–3949
Sheikh J, Juneja H, Ingle V, Ali P, Ben Hadda T (2011) Synthesis and in vitro Biology of Co(II), Ni(II), Cu(II) and Zinc(II) complexes of functionalized beta-diketone bearing energy buried potential antibacterial and antiviral O,O pharmacophore sites. J Saudi Chem Soc. doi:10.1016/j.jscs.2011.04.004
Shekarchi M, Pirali-Hamedani M, Navidpour L, Adib N, Shafiee A (2008) Synthesis, antibacterial and antifungal activities of 3-Aryl-5-(pyridin-3-yl)-4,5-dihydropyrazole-1-carbothioamide derivatives. J Iran Chem Soc 5:150–158
Shoman ME, Abdel-Aziz M, Aly OM, Farag HH, Morsy MA (2009) Synthesis and investigation of anti-inflammatory activity and gastric ulcerogenicity of novel nitric oxide-donating pyrazoline derivatives. Eur J Med Chem 44:3068–3076
Siddiqui AA, Rahman MA, Shaharyar M, Mishra R (2010) Synthesis and anticonvulsant activity of some substituted 3,5-diphenyl-2-pyrazoline-1-carboxamide derivatives. Chem Sci J 8:2–10. http://astonjournals.com/csj
Simovic D, Di M, Marks V, Chatfield DC, Rein KS (2007) 1,3-dipolar cylcloadditions of trimethylsilyldiazomethane, revisited: steric demand of the dipolarophile and the influence on product distribution. J Org Chem 72:650–653
Soni N, Pande K, Kalsi R, Gupta TK, Parmar SS, Barthwal JP (1987) Inhibition of rat brain monoamine oxidase and succinic dehydrogenase by anticonvulsant pyrazolines. Res Commun Chem Pathol Pharmacol 56:129
Turan-Zitouni G, Chevallet P, Kilic FS, Erol K (2000) Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 35:635
Yar MS, Siddiqui AA, Ali MA (2006) Synthesis and evaluation of phenoxy acetic acid derivatives as anti-mycobacterial agents. Bioorg Med Chem Lett 16:4571–4574
Acknowledgments
Prof. T. Ben Hadda would like to thank the ACTELION; the Biopharmaceutical Company of Swiss, for the on-line molecular properties calculations and to Ministry of High Science and Education of Rabat (Morocco) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadda, T.B., Ali, M.A., Masand, V. et al. Tautomeric origin of dual effects of N1-nicotinoyl-3-(4′-hydroxy-3′-methyl phenyl)-5-[(sub)phenyl]-2-pyrazolines on bacterial and viral strains: POM analyses as new efficient bioinformatics’ platform to predict and optimize bioactivity of drugs. Med Chem Res 22, 1438–1449 (2013). https://doi.org/10.1007/s00044-012-0143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0143-6