Abstract
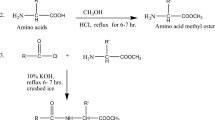
Mutual prodrugs consisting of mefenamic acid with menthol and thymol have been synthesized as a gastrosparing NSAIDs, devoid of ulcerogenic side effects. The structures of synthesized esters were confirmed by IR, 1H NMR, and mass spectroscopy. The kinetics of ester hydrolysis was studied in nonenzymatic buffer solutions, at pH 2 and 7.4 as well as in human plasma by HPLC. Its anti-inflammatory, analgesic, and ulcerogenic activities were evaluated. Then biochemical parameters (GWM and Hexosamine), oxidative parameters (LPO, GSH, CAT, and SOD), and protein estimation was also done. The results indicated that synthesized prodrugs are chemically stable, biolabile, and posses optimum lipophilicity. The synthesized prodrugs are characterized by better ulcer index than the parent drug.

Similar content being viewed by others
References
Abraham M, Rudolph MD (1981) Effects of aspirin and acetaminophen in pregnancy and in the newborn. Arch Inter Med 141(3):358–363. doi:10.1001/archinte.1981.00340030090016
Bandyopadhyay U, Das D, Banerjee RK (1999) Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci 77:658–665
Blumenkrantz N, Asboe-Hansen G (1976) An assay for total hexosamine and a differential assay for glucosamine and galactosamine. Clin Biochem 9(6):269–274. doi:10.1016/S0009-9120(76)80075-6
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ana Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Corne SJ, Morrissey SM, Woods RJ (1974) Proceedings: a method for the quantitative estimation of gastric barrier mucus. J Physiol 242:116–117
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophy 82:70–77. doi:10.1016/0003-9861(59)90090-6
Gibson T (1988) Nonsteroidal anti-inflammatory drugs: another look. Br J Rheumatol 27:87–90. doi:10.1093/rheumatology/27.2.87
Hassan A, Martin E, Puig-Parellada P (1998) Role of antioxidants in gastric mucosal damage induced by indomethacin in rats. Methods Find Exp Clin Pharmacol 20:849–854. doi:10.1358/mf.1998.20.10.487540
Jain NK, Patil CS, Kartasasmita RE, Decker M, Lehmann J, Kulkarni SK (2004) Pharmacological studies on nitro-naproxen (naproxen-2-nitroxyethylester). Drug Dev Res 61:66–78. doi:10.1002/ddr.10337
Koster R, Anderson M, De Beer EJ (1959) Acetic acid for analgesic screening. Fed Proc 18:412–426
Laporte JR, Carne X, Vidal X, Moreno V, Juan J (1991) Upper gastrointestinal bleeding in relation to previous use of analgesics and non-steroidal anti-inflammatory drugs. Catalan countries study on upper gastrointestinal bleeding. Lancet 337(8733):85–89. doi:10.1016/0140-6736(91)90744-A
Madhukar M, Sawraj S, Sharma PD (2010) Design, synthesis and evaluation of mutual prodrug of 4-biphenylacetic acid and quercetin tetramethyl ether (BPA-QTME) as gastrosparing NSAID. Eu J Med Chem 45:2591–2596. doi:10.1016/j.ejmech.2010.02.047
Menon B, Sharma PD (2009) Design, synthesis and evaluation of diclofenac-antioxidant mutual prodrugs as safer NSAIDs. Ind J Chem 48 B:1279–1287
Moser P, Sallmann A, Wiesenberg I (1990) Synthesis and quantitative structure-activity relationships of diclofenac analogs. J Med Chem 33(9):2358–2368. doi:10.1021/jm00171a008
Neises B, Steglich W (1978) Simple method for the esterification of carboxylic acids. Angew Chem Int Ed 17:522–524. doi:10.1002/anie.197805221
Pérez Gutthann S, García Rodríguez LA, Raiford DS, Duque Oliart A, Ris Romeu J (1996) Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure. Arch Inter Med 156(21):2433–2439. doi:10.1001/archinte.1996.00440200041005
Piacham T, Isarankura-Na-Ayudhya C, Nantasenamat C, Yainoy S, Ye L, Bülow L, Prachayasittikul V (2006) Metalloantibiotic Mn(II)-bacitracin complex mimicking manganese superoxide dismutase. Biochem and Biophy Res Commun 341:925–930. doi:10.1016/j.bbrc.2006.01.045
Sairam K, Rao CV, Dorababu M, Goel RK (2001) Prophylactic and curative effects of Bacopa monniera in gastric ulcer models: Abstract. Phytomed 8:423–430. doi:10.1078/S0944-7113(04)70060-4
Utley HG, Bernheim F, Hochstein P (1967) Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophy 118:29–32. doi:10.1016/0003-9861(67)90273-1
Vane JR, Botting RM (1995) New insight into the mode of action of anti-inflammatory drugs. Inflamm Res 44:1–10. doi:10.1007/BF01630479
Winter CA, Risley EA, Nuss GW (1962) Carrageenan-induced edema in hind paw of the rat as an assay for anti inflammatory drugs. Proc Soc Exp Bio Med 111:544–547
Wynne HA, Long A, Nicholson E, Ward A, Keir D (1998) Are altered pharmacokinetics of non-steroidal anti-inflammatory drugs (NSAIDs) a risk factor for gastrointestinal bleeding? Br J Clin Pharmacol 45(4):405–408. doi:10.1046/j.1365-2125.1998.t01-1-00696.x
Yalkowsky SH, Morozowich W (1980) A physical chemical basis for the design of orally active prodrugs. In: Ariens EJ (ed) Drug design. Academic press, New York, pp 121–135
Zuckner J (1986) International experience with diclofenac in rheumatoid arthritis. Am J Med 80(4B):39–42. doi:10.1016/0002-9343(86)90078-1
Acknowledgments
Authors thank M/S Zydus Cadila, Ahmedabad, Gujarat, India for providing mefenamic acid as gift sample and to Vice Chancellor, GLA University, Mathura UP, India for providing the research facilities to carry out this work in the Institute of Pharmaceutical Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, K., Shrivastava, S.K. & Mishra, P. Evaluation of mefenamic acid mutual prodrugs. Med Chem Res 22, 70–77 (2013). https://doi.org/10.1007/s00044-012-0016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0016-z