Abstract
This study investigated the influence of dissolved organic matter (DOM) additions on phosphate sorption kinetics of iron-rich sediments (39–50% hematite and goethite) from an ephemeral stream in the arid Pilbara region of sub-tropical northwest Australia. While phosphate sorption in stream sediments is known to be strongly influenced by sediment mineralogy as well as interactions with DOM, the mechanisms and significance of DOM on P-release from sediments with high sorption capacities, are largely undescribed. We assessed phosphorus (P) sorption behaviours by adding a range of solutions of known inorganic P concentrations that were amended with variable loadings of DOM derived from leachates of leaf litter to sediments from stream pools during the non-flowing phase. We compared the sorption capacity of the sediments and concurrent changes in DOM composition measured using fluorescence spectroscopy. We show that the low-dose DOM addition (~ 4 mg L−1 DOC) had the effect of reducing sediment P adsorption capacity, while for the high-dose DOM addition (~ 45 mg L−1 DOC), it was increased. The high-dose DOM was similar to pore water DOC and likely saturated sediment surface adsorption sites and produced P–OM–Fe complexes. This resulted in increased removal of P from solution. Sediment P sorption characteristics were well fitted to both Freundlich and Langmuir isotherm models regardless of DOC concentration. Langmuir P sorption maxima ranged from 0.106 to 0.152 mg g−1. General P sorption characteristics of these iron-rich sediments did not differ among pools of contrasting hydrological connectivity. Our results show how humic-rich DOM can modulate the sediment P availability in dryland streams. Unravelling the complexities of P availability is of particular significance to further our understanding of biogeochemical processes in aquatic ecosystems where P often acts as a limiting nutrient.
Similar content being viewed by others
Introduction
Phosphorus (P) sorption kinetics of stream sediments play a key role in regulating P content of the water column and are recognised to have a strong influence on ecological processes within aquatic systems (Reddy et al. 1999). Competition for limited P resources between biotic and abiotic components of freshwater streams is increased when sediments are dominated by iron-rich minerals (Fink et al. 2016). However, dissolved organic matter (DOM) content and composition can also influence P sorption behaviours of stream sediments (McDowell and Sharpley 2001), albeit in inconsistent and complex ways (Verbeeck et al. 2017; Wang et al. 2008). Under anaerobic conditions, organic matter enhances microbially mediated reductive dissolution of iron (III) minerals and subsequently releases the phosphate ion (Mitchell and Baldwin 1998; Watts 2000a). In contrast, aerobic oxidation of iron (II) back to iron (III) produces amorphous iron (III) oxyhydroxides with high binding capacity, which in turn enables the recovery (sorption) of a portion of the P released (Howitt et al. 2008; Verbeeck et al. 2017; Wang et al. 2008). Thus, changes in redox conditions, including from wet–dry cycles resulting from variable flow regimes, will impact P–DOM–Fe interactions in stream sediments. DOM can also inhibit phosphate sorption to ferric sediments through the occupation and blocking of mineral surface sites (Gu et al. 1995). DOM might also act as a chelating agent in iron-rich sediments capable of interacting with both the electron acceptors on sediment surfaces ‘freeing’ iron and phosphate into solution (Baken et al. 2016; Daugherty et al. 2017). We might, therefore, expect that the relative amounts and compositions of autochthonous sources of DOM (e.g. proteins from algae) versus allochthonous sources (dominated by aromatic structures and high molecular weight compounds) to also be important moderators of the ways that P and iron interact.
Recent studies have demonstrated that the concentration and composition of DOM of ephemeral streams are strongly influenced by the dynamics of both surface water and shallow alluvial groundwater (Fellman et al. 2011; Harjung et al. 2018; Siebers et al. 2016). Allochthonous inputs from riparian vegetation make a significant contribution to sediment organic matter loads of intermittent and ephemeral streams, as well as contribute to DOM in the water column and hyporheic zone (Baldwin 1999; Datry et al. 2018; Fellman et al. 2013). Other minor sources of DOM from the catchment such as soil organic matter, algae, and bacteria may also be present but are considered to not contribute significantly to the overall DOM stock. Litter (leaves and small branch material), as well as coarse woody debris, may enter reaches and isolated pools directly from overhanging riparian vegetation or can be washed into streams from the surrounding catchment during rainfall and flood events (Gonçalves et al. 2014; Tonin et al. 2017). Direct input of litterfall from overhanging riparian vegetation is especially important in dryland regions where the stream corridor—and particularly around more persistent pools—is much more productive than the surrounding catchment. This tannin-rich litter releases significant amounts of DOM in to the water column through the leaching of fresh litter, as well from decomposing material. This DOM can then be further concentrated as surface water contracts during drought (Harjung et al. 2018; Siebers et al. 2016). Drying has been found to enhance processing of DOM in the hyporheic zone of intermittent Mediterranean streams, with preferential retention of higher molecular weight fractions at the hyporheic interface with alluvial groundwater (Harjung et al. 2018).
Sediments and soils that are consistently exposed to drying and rewetting cycles tend to release more P than if consistently moist (Baldwin 1996; Grierson et al. 1998). Drying and rewetting cycles also decrease the P affinity of streambank sediments compared to sediments that remain permanently inundated (Watts 2000b). Sediments in ephemeral stream channels are exposed to varying degrees of drying and for differing periods of time—sometimes years—owing to the contraction and fragmentation of surface water to isolated remnant pools. Drying of sediments may decrease P adsorption capacity (Attygalla et al. 2016), while P desorption and release from sediments and soils is enhanced upon rewetting (Baldwin 1996; Grierson et al 1998; Kerr et al. 2010; Knowles et al. 2012). These effects can persist for extended periods of time—a study of wetland clays and silts showed P adsorption capacity decreased for several weeks after rewetting (Song et al. 2007). Baldwin and Mitchell (2000) have also shown that wetland sediments that go through decadal wetting–drying cycles have reduced adsorption capacity even when the current inundation period has spanned many years. However, stream channel beds tend to be more coarsely textured than wetland soils. Further, there is also high heterogeneity in exposure to the extent of drying and rewetting within channels of ephemeral and intermittent streams owing to varying levels of connectivity to alluvial groundwater (Fellman et al. 2011; Siebers et al. 2016). This process of cyclical drying/rewetting in association with increased DOM concentrations in individual pools and different forms of DOM, allow for the production of P–OM–Fe complexes, which may enable P to remain suspended in solution as colloidal particles (Baken et al. 2016; Yan et al. 2017), or form more complex structures that adsorb to sediment mineral surfaces (Cheng et al. 2004). We might thus expect that stream sediments in reaches that are less connected to groundwater and, therefore, dry out more frequently and for longer periods of time, to show reduced P sorption capacity and different sorption behaviours in association with DOM, compared to sediments that remain persistently inundated.
In this study, we sought to characterise the P sorption behaviours of iron-rich sediments from pools along an ephemeral stream in the arid Pilbara region of northwest Australia. While phosphate sorption in stream sediments is known to be strongly influenced by sediment mineralogy as well as interactions with DOM, the mechanisms and significance of DOM on P-release from sediments with high sorption capacities is poorly understood. Particularly for ephemeral stream systems in semi-arid and arid environments worldwide, we use laboratory P sorption experiments coupled with fluorescence spectroscopy to characterise chemical composition and bioavailability of DOM to assess how DOM derived from leaf litter leachates (as a predominant allochthonous source) influenced P sorption. We expected that an increase in DOM concentration would decrease the P adsorption capacity of sediments and increase P desorption from sediments. We further expected that more aromatic-like DOM structures—such as humic acids—play a major role in mediating DOM–sediment–P interactions.
Methods
Study site and sampling
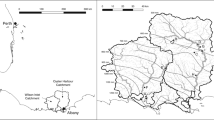
Sediments and leaf litter samples were collected from six pools along 8 km of Coondiner Creek (− 23.00 °S, 119.62 °E), an ephemeral stream in the Hamersley Ranges of northwest Australia (Fig. 1). Coondiner Creek is considered representative of many streams in the region that cut through a network of semi-confined gorges throughout the ranges (Fellman et al. 2011; Siebers et al. 2016). Stream bed sediments are primarily reworked channel iron deposits, containing a high proportion (50–60%) of Fe-hydroxide minerals, primarily hematite and goethite (Ramanaidou et al. 2003).
The regional climate is sub-tropical and semi-arid to arid with a mean annual rainfall of ~ 300 mm that is highly variable both within and among years. Rainfall occurs predominantly in the austral summer ‘wet season’ arising from cyclones, monsoonal lows and tropical thunderstorms, which punctuate months and sometimes years of prolonged drought (Bureau of Meteorology, 2018). Mean daily temperatures range from minima and maxima of 25 to 39 °C in the summer, and from 8 to 22 °C in the winter, with annual pan evaporation 1200 to 2000 mm (Charles et al. 2015).
The flow regime of Coondiner Creek is directly linked to rainfall dynamics, with seasonal discharge during the wet summer months (December to March) but with surface flow only occurring in years with large rainfall events (Dogramaci et al. 2015; Rouillard et al. 2015). The streams in this region are extremely intermittent and highly ephemeral (Kennard et al. 2010). During the ‘dry season’ (April to November), and in years with no cyclone activity, surface waterways become disconnected to form a chain of pools along drainage lines or dry out completely (Beesley and Prince 2010; Fellman et al. 2011; Siebers et al. 2016). Groundwater plays an important role in maintaining surface water volume in many of these stream pools throughout the catchment (Dogramaci et al. 2012).
Coondiner Creek was sampled during the relatively cooler dry season in June 2016, when surface flow had ceased and the stream had contracted to a series of disconnected pools. Pools were classed as persistent (n = 3) where alluvial throughflow maintains water level in the pool throughout the year, or ephemeral (n = 3) where pools are disconnected from the alluvial groundwater and hence pools frequently dry out in the weeks to months following flow cessation, exposing sediments to drying. Pool classifications were based on previous hydrological studies of the stream (Fellman et al. 2011), and through confirmation of their hydrologic status based on their water isotope composition at the time of sampling (data not shown but see Dogramaci et al. (2015) and Iles (2019) for further explanation). Sediments (0–5 cm depth) were collected using a hand corer from three positions within each pool at ~ 40–50 cm water depth and generally within 2 m of the pool edge. The sediment samples from each pool were passed through a 2 mm sieve. The < 2 mm fractions were then combined to provide one representative sample for assessing P sorption characteristics per pool. Sediments were sealed in plastic ‘zip-lock’ bags and then packed in a portable cooler for transport to the laboratory. All sediments were then oven-dried (30 °C) for 72 h in preparation for adsorption–desorption experiments. Aged leaves were selected from leaf litter of the most dominant riparian tree species, Eucalyptus camaldulensis subsp. refulgens, adjacent to the pools to prepare DOM extracts. Litter leachates were prepared by gently agitating 20 g leaf material in 1 L MilliQ water for 2 h on a shaker table (Fellman et al. 2013). The leachate was then filtered (Whatman GF/F 0.7 µm) to exclude the bulk of the particulate organic matter and retain the dissolved components. The leachate was prepared the day before batch experiments were performed and stored in the dark wrapped in foil and refrigerated prior to use.
Sediment mineralogy and elemental chemistry
Sediment pH and electrical conductivity (EC) were measured in 1:10 (w/v) sediment:solution with a bench top pH meter (Orion model 520A) and EC probe (YSI model 85). A subsample of each sediment was dried (50 °C) and ground to a fine powder (< 60 µm) for mineralogical and chemical analysis. OH–extractable inorganic P (OH–Pi) and OH–extractable total P (OH–Pt) were measured after extraction in 0.1 M NaOH (Bowman and Cole 1978). Extracts were centrifuged at 10,000 rpm for 5 min. A ~ 10 mL aliquot of supernatant was then acidified with 1 mL of concentrated HCl for the measurement of OH–Pi. OH–Pt was determined after an aliquot of unacidified extract was evaporated on a digestion block at 120 °C and then digested in concentrated H2SO4/H2O2 at 340 °C. Phosphorus concentrations in OH–Pi and OH–Pt extracts were then measured using the modified ascorbic acid method (Kuo 1996; Murphy and Riley 1962) and concentration reported in µg P g−1 (dry weight). Sediment geochemical characterisation was determined by X-ray fluorescence (XRF) by Intertek Genalysis (Perth, WA). A fused disk of the powdered sample was prepared with borate flux. Loss on ignition (LOI) was determined after combustion at 425 °C, 650 °C, and 1000 °C by thermogravimetric analysis. All LOI values are reported as weight percent (wt%). Sediment crystalline structure and amorphous content was determined by quantitative X-ray diffraction (XRD) (PANalytical Cubix3, Almelo, Netherlands). Samples were packed and presented as un-orientated powder mounts. ZnO and CaF2 internal standards were added to each sample and amorphous content was determined by an internal standard scan.
Phosphorus sorption characteristics
Phosphorus (P) adsorption experiments were undertaken by adding a range of known P concentrations that have been shown previously as appropriate for sediments that are likely to have high P sorption capacity (Bentley et al. 1999; Nair and Reddy 2013; Pant and Reddy 2001). Phosphate solutions were prepared in a 0.003 M KCl buffer. Higher concentrations of salt can impact (decrease) water solubility of organic matter adhered to soil particles (Turner and Rawling 2001). We were thus fairly conservative in the buffer that we used. In the samples with no DOM additions (DOC0), 5 g sediment was weighed into 125 mL high-density polyethylene (HDPE) bottles containing 100 mL of phosphate solution (0, 2.5, 5, 10, 20, 40, or 100 mg L−1 in 0.003 M KCl). Solution blanks were included in all steps of the procedure to assess P recovery and ensure P was not sorbed to the HDPE bottle. Samples were mixed on an end-over-end shaker at 22 °C (± 2 °C) for 24 h. Sediments were allowed to settle for 30 min prior to the supernatant being filtered (Sartorius minisart 0.45 µm). P concentrations in the filtered supernatant were then analysed using a modified ascorbic acid method (Kuo 1996; Murphy and Riley 1962).
To investigate the effects of dissolved organic matter (DOM) on P adsorption characteristics, the above procedure was repeated on two further subsets of sediments by adding eucalypt litter leachates with (i) DOC concentrations of 3.7 mg L−1 (DOC4), or (ii) DOC concentrations of 44.6 mg L−1 (DOC45). The two DOC treatment concentrations were selected based on prior observations of DOC concentrations in pools of Coondiner Creek, generally in the range of 3–6 mg L−1 (Siebers et al. 2016). We used a tenfold increase to emulate the expected higher DOC concentrations encountered in sediment pore water and which would be expected in the hyporheic zone (Harjung et al. 2018), especially in pools that accumulate organic matter. The concentration of inorganic P in leaf litter leachates was deemed insignificant (i.e. µg L−1) compared to the high concentrations of inorganic P we were adding (mg L−1) during the adsorption experiments. P concentrations of the filtered supernatant were analysed as described earlier.
DOM remaining in solution for a subset of P treatments following batch adsorption experiments was characterised in order to assess if particular components (e.g. fulvic vs humic-like) were more likely to be sorbed than others. Differences in the optical absorption and DOM fluorescence properties (methods for characterisation are described in detail as follows) among the three treatments (DOC0, DOC4, and DOC45) were thus investigated on a subset of samples (initial P concentration (Ci) = 0, 40, 100 mg L−1).
The amount of P sorbed was calculated as
where qe is the ratio of P sorbed by sediment (mg g−1), Ci and Ce are the initial and equilibrium P concentrations (mg L−1), V is the solution volume (L) and W is the weight of sediment (g). Plotting the amount of P sorbed (qe) vs equilibrium concentration (Ce) we examined the linear region of the curve (Ce < 10 mg L−1):
Here, the slope is equal to the linear adsorption coefficient (Kl), and intercept (b) equal to initial soil P. The equilibrium P concentration (EPC0) was then calculated:
The experimental data were fitted to both Langmuir and Freundlich isotherm models using non-linear least squares. The Freundlich equation is as follows (Freundlich 1907):
where KF (L mg−1) is a constant related to adsorption energy, and n is a correction factor.
The Langmuir equation (Langmuir 1918) used was
where KL (L mg−1) is the Langmuir isotherm constant, and b (mg g−1) is the maximum adsorption capacity, often referred to as ‘Smax’ (Pant and Reddy 2001).
P desorption was measured at the conclusion of the incubations. For each of the three treatments (DOC0, DOC4, and DOC45), the supernatant was discarded and the sediment retained. Then, 100 mL 0.003 M KCl was added to each bottle and the sediment shaken for 24 h. Samples where then filtered (0.45 µm) and the supernatant analysed for P in solution as previously described.
Dissolved organic matter characterisation
DOM remaining in solution for a subset of P treatments following batch adsorption experiments was characterised in order to assess if particular components of DOM were more likely to be sorbed than others. These subset treatments with initial P concentration (Ci) of 0, 40 and 100 mg P L−1 were further analysed by absorbance and fluorescence spectroscopy. Absorbance spectra 200 to 800 nm were measured on a UV–visible spectrophotometer (Varian Cary 50 Probe). Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) were measured simultaneously on a Shimadzu TOC-V analyser coupled with a total nitrogen module (Shimadzu TNM-1). C:N ratios of the equilibrated solutions were calculated as the weight ratios of DOC:TDN. Specific UV absorbance at 254 nm (SUVA254) (Weishaar et al. 2003) and spectral slope (S275–295) (Helms et al. 2008) were calculated from the absorbance spectra.
DOM fluorescence was measured on a Varian Cary Eclipse spectrofluorometer (Varian Medical Systems, Inc. California USA). Extracts were diluted to within optical range with an auto-dilutor (Hamilton, Microlab) to avoid self-quenching due to inner-filter effects (Ohno 2002). Excitation emission spectra (EEM) were produced for excitation wavelengths 240 to 450 nm at 5 nm intervals with emission intensities captured from 300 to 600 nm at 2 nm intervals. All fluorescence excitation–emission matrices (EEMs) were corrected for instrument bias using instrument correction files and normalised to Raman Units (R.U.) by the area under the Raman water peak at Ex350 nm. Humification index (HIX) was calculated following the methods outlined in Ohno (2002). The main contributing fluorescing organic matter components of resulting EEMs were extracted and quantified via parallel factor analysis (Murphy et al. 2013; Stedmon and Bro 2008).
Data analyses
Non-metric multidimensional scaling (nMDS) of sediment characteristics to assess similarity among pools was performed following normalisation, and quantified with permutational multivariate analysis of variance (PERMANOVA) in Primer (Primer v 6.1.18 & PERMANOVA + v 1.0.8). Sorption model fitting and statistical analysis were performed in R version 3.4.1 (R Core Team 2017). Adjusted R-squared values and residual sum of squares (RSS) were assessed to support sorption model selection. Parallel factor analysis (PARAFAC) was performed in MATLAB (R2012a) using the n-way and drEEM (v4.0) toolboxes (Murphy et al. 2013). The PARAFAC model was trained to look for best fit between three to seven fluorophore components within the experimental data. The four-component (C1–C4) model was validated using split-half analysis (Stedmon and Bro 2008), model components were compared to other fluorophore components in the OpenFluor spectral library (Murphy et al. 2014) with OpenChrom (v1.3.0 Dalton). Peaks are presented as maximum fluorescence intensity (Fmax) values in Raman units for each component. Finally, differences in DOM components and indices with pool hydrology, DOM treatment, and initial P concentration were investigated with PERMANOVA routines of Fmax values.
Results
Sediment properties
A summary of sediment characteristics is provided in Table 1. Overall, sediments collected from pools along Coondiner Creek were iron-rich and similar in character irrespective of hydrological regime (i.e. there was no mineralogical difference between ‘persistent’ versus ‘ephemeral’ pools). The OH–extractable inorganic P (OH–Pi) (0.2–1.0 µg P g−1) and total P (OH–Pt) (70–82 µg P g−1) were also similar among pools (Table 1), with the exception of ‘Pool W’. Pool W had elevated P concentrations (OH–Pi: 4.38, OH–Pt: 302 µg P g−1); in contrast to the other pools sampled, this pool was located about 50 m from the main channel, and appears to be maintained by runoff from a small side tributary rather than replenishment via surface or groundwater flows in the creek. The < 2 mm fraction of Coondiner Creek sediments consisted predominantly of sand, with less than 1% fines (silt and clay).
Elemental XRF showed sediments from all pools were predominantly composed of iron and aluminium oxides (Fe 26–42%, Al2O3 5–10%) (Table 1). XRD analysis showed sediment mineralogy was primarily composed of hematite (20–37 wt%) and goethite (10–16 wt%), along with quartz (11–23 wt%). Sediment LOI ranged from 2.5 to 3.2% at 425 °C with total LOI 4.3–5.0% at 1000 °C. Comparison between sediments XRD from ‘persistent’ and ‘ephemeral’ pools indicated that their geochemistry did not differ with pool hydrology (Fig. 2); centroids and dispersion between groups were no greater than within groups for both XRF chemical characterisation (PERMANOVA: Pseudo-F = 0.689, df = 1/4, P = 0.69), and XRD crystalline and amorphous content (PERMANOVA: Pseudo-F = 2.159, df = 1/4, P = 0.31).
Fluorescent properties of litter leachates and sediment extracts
PARAFAC decomposition of EEM spectra identified four fluorescing components in the DOM dataset (Fig. 3). Component C1 had excitation maxima of 310 and 400 nm with emission maxima 420–444 nm. Component C2 had excitation maxima of 255, 340, and 445 nm and emission maxima of 464–502 nm. Components C1 and C2 were matched in the OpenFluor library with humic-like components C1 and C2 from Baker et al. (2014) (similarity r2 = 0.93 and 0.92). Component C3 had excitation maxima of 280 and 390 nm and emission maxima 306–356 nm. Component C3 was matched with the tryptophan-like C5 from Baker et al. (2014) (similarity r2 = 0.90). Component C4 had excitation maxima of 250 nm and emission maxima of 304 and was matched with tyrosine-like C6 from Shutova et al. (2014) (similarity r2 = 0.93). The leaf litter leachates used in the experiments consisted of humic-like C1 and C2 peaks and a tryptophan-like C3 peak. Tyrosine-like C4 was absent from the leachate. The sediments naturally contained small amounts of all four components. Sediment extracts exhibited primarily components C1 and C4 with trace amounts of C2 and C3 also present.
Fluorescent DOM components derived from fluorescence spectroscopy and PARAFAC analysis. a Modelled excitation-emission spectra of humic-like components 1 and 2, tryptophan-like component 3, and tyrosine-like component 4. b Excitation (red dash) and emission (blue line) spectral loading of each corresponding component
Phosphorus sorption characteristics
Phosphorus sorption characteristics for sediments are presented in Table 2. Sorption characteristics from all pools were generally best described by the Freundlich model (Table 3; Fig. 4), although there were some exceptions where a Langmuir model provided a better fit (e.g. persistent pools were more similar to the Langmuir model when DOM was present; Table 3). Results for both Freundlich and Langmuir isotherms are thus reported for comparison.
Experimental data from batch phosphorus adsorption experiments. Mean adsorption (qe) and standard error (n = 3) for sediments from ‘persistent’ (black square) and ‘ephemeral’ (shaded circle) pools versus equilibrium P concentration (Ce) shown. The data were fitted to Freundlich (dashed line) and Langmuir (solid line) isotherms. Each of the models was fitted to ‘persistent’ (black line) and ‘ephemeral’ (shaded line) pools
The Freundlich adsorption energy constant KF ranged from 0.024 to 0.049 L mg−1. There was no difference in KF values among DOM treatments or with pool hydrology. ‘Persistent’ and ‘ephemeral’ pools also did not differ in sorption characteristics (Table 3). However, the Freundlich correction factor (n) was significantly higher in the DOC4 treatment (2.364–3.157) compared to both the DOC0 (1.705–2.192) and DOC45 (1.952–2.278) treatments (two-way ANOVA: P = 0.002).
The Langmuir model generally fitted adsorption data where Ce < 50 mg L−1, but was less accurate at higher concentrations and thus underestimated maximum sorption capacity (Fig. 4). The Langmuir isotherm constant (KL) ranged from 0.386 to 1.012 L mg−1 (Table 3). There was no difference in KL values between DOM treatments or hydrology. Langmuir adsorption capacity (b) ranged from 0.106 to 0.152 mg g−1. P adsorption capacity was significantly lower in the DOC4 treatment (0.106–0.138 mg g−1) compared to DOC0 (0.141–0.149 mg g−1) and DOC45 (0.114–0.152 mg g−1) (two-way ANOVA: P = 0.004). ‘Persistent’ and ‘ephemeral’ pools did not differ in sorption characteristics and there was no interaction between hydrology and DOM.
Desorption of P from iron-rich sediments
The amount of P desorbed after 24 h ranged from 0 to 0.05 mg g−1 across the three DOM treatments, demonstrating that much of the added P is retained by the Fe-rich sediment once adsorbed (Fig. 5). However, P desorption from sediments also increased with the amount of P previously adsorbed. Increasing the amount of DOM in the sediment–water mix generally increased the amount of P desorbed from sediment. A linear fit to the experimental data indicated that the amount desorbed was 10.0–12.7% (Pearson’s r = 0.98, 0.87) for the DOC0 treatment, 11.7–16.4% (r = 0.35, 0.49) for DOC4, and 17.2–23.9% (r = 0.69, 0.95) for DOC45. However, when DOM5 was added, nearly all the P sorbed up to 0.1 mg g−1 was retained by the sediment (Fig. 5). In this case, significant desorption occurs only once the sediment P concentration exceeded 0.1 mg g−1.
Changes in DOM composition with incubation and P adsorption
Based on extensive prior studies of DOM in the Coondiner Creek catchment (Fellman et al. 2011; Siebers 2015; Siebers et al. 2020), DOM in solution at the beginning and end of 24 h incubation in the DOC0 treatment was assumed to be below our DOC analytical detection limit (4 µg L−1). Hence, carbon-based parameters are reported only for DOC4 and DOC45 treatments (Fig. 6). Interestingly, the DOC concentration remaining in solution following batch adsorption experiments was higher for ‘persistent’ pools than ‘ephemeral’ pools in both DOC4 and DOC45 treatments. However, there was no clear relationship between initial P concentration (Ci) and final DOC concentration. The C:N ratio of the equilibrated solution was positively correlated with the initial P concentration up to 20 mg P L−1 for the DOC45 treatment but at initial P concentrations greater than 20 mg L−1, the C:N ratio of the equilibrated solution remained constant (Fig. 6). SUVA254 values following batch adsorption experiments ranged from 0.35 to 1.20 L mg−1 m−1. The SUVA254 value increased with increasing initial P concentration for the range 0 to 20 mg P L−1 for both the DOC4 and DOC45 treatments (Fig. 6).
Measured at the conclusion of batch phosphorus adsorption experiments. Dissolved organic carbon (DOC), C:N ratio, and Specific UV absorbance at 254 nm (SUVA254), Values are means with standard error (n = 3) for sediments from ‘persistent’ (black square) and ‘ephemeral’ (shaded circle) pools. Note different scales on y-axis between DOC panels
Absorption spectra indicate that the composition of DOM remaining in solution at the end of the incubation correlates to the initial P concentration (Fig. 7). The spectral slope value (S275–295) increased with increasing P concentration (Fig. 7). There was an initial rapid increase in slope value as initial P increased from 0 to 10 mg L−1 with spectral slopes levelling out at higher initial P concentrations. The humification index (HIX) ranged from 0.46 to 0.81 (Fig. 7).
UV–Vis and Fluorescence indices measured at the conclusion of batch phosphorus adsorption experiments. Spectral slope (S275–295) and humification index (HIX) values are presented as means with standard error (n = 3) for sediments from ‘persistent’ (black square) and ‘ephemeral’ (shaded circle) pools. Note: spectral slope was not calculated for persistent DOC0 treatments as absorbance was only measured at 254 nm for these samples
PARAFAC analysis of the fluorescence EEM spectra split the experimental dataset into four DOM components. The fluorescence maxima for all DOM fluorescing components were reflective of the DOC concentration of the initial DOM treatment additions (Fig. 8). Fmax values were significantly different between DOM treatments (F2,45 = 56.82, P = 0.001), with DOC45 > DOC4 > DOC0. Component correlation plots while validating the model, also indicated that Fmax values for humic-like C1 and C2 components were highly correlated (Pearson’s r = 0.94). Fluorescence maxima (Fmax) at the end of 24 h batch phosphorus adsorption experiments for sediments from ‘persistent’ and ‘ephemeral’ pools indicated that humic acids (components C1 and C2) were desorbed from sediments, particularly when initial P concentration was high (Fig. 8). Fluorescence maxima for these components significantly increased with increasing initial P concentration (C1: F2,45 = 4.88, P = 0.006, C2: F2,45 = 23.61, P = 0.001). There was also an interaction between initial P and DOM treatment (C1: F4,45 = 4.55, P = 0.003, C2: F4,45 = 7.99, P = 0.001). Tryptophan-like C3 fluorescence maxima did not change with increasing initial P concentration (F2,45 = 0.09, P > 0.05) and there was no interaction between initial P and DOM treatment for this component (F4,45 = 2.18, P > 0.05). Tyrosine-like C4 appears to be either a degradation product or extracted OM from the sediments as this component was not present in the initial DOM leachates (Fig. 8). Fluorescence maxima significantly increased with increasing initial P concentration (F2,45 = 4.71, P = 0.017) and there was a significant interaction between initial P and DOM treatment (F4,45 = 11.4, P = 0.001).
Fluorescence maxima (Fmax) for PARAFAC-derived DOM components at the end of 24 h batch phosphorus adsorption experiments for sediments from ‘persistent’ (black) and ‘ephemeral’ (shaded) pools. Components 1 and 2 are humic-like fluorophores, and component 3 is protein-like (amino acids), while component 4 is thought to be a sediment derived OM degradation product. Mean and standard error (n = 3)
Discussion
This study provides analysis of P sorption behaviours of sediments from a sub-tropical ephemeral stream. Sorption capacity and behaviours were overwhelmingly controlled by the iron-rich and highly weathered nature of the sediments, which are ubiquitous throughout the catchment. Most importantly, the results of this study illustrate the complex ways that DOM may interact with iron-rich sediments to influence P availability. Our findings also suggest that both P adsorption and desorption processes are important for mediating the concentrations of potentially biologically ‘available’ P in the water column and likely the hyporheic zone. While the iron-rich sediments examined in this study act as a sink for P, under field conditions the concentration of DOM in the water column and in sediment pore water are highly likely to play a role in regulating P desorption, especially during the protracted periods of zero surface flows that are characteristic of ephemeral stream systems. In particular, the addition of DOM may increase P desorption from sediments when sediment P loading was high (i.e. Pads is above saturation). However, DOM desorption appears to increase at high P loadings, which suggests in more eutrophic settings (e.g. when nutrients become concentrated in pools as they dry or if there are external inputs of P), that DOM may be displaced into the water column. However, in this study, only the physical desorption or adsorption are addressed, microorganisms are also likely to play a role in P-release and, therefore, availability. Below, we discuss in further detail the mechanisms underpinning our observations and their significance within the hydrodynamic settings of our study stream.
P sorption behaviours of Fe-rich sediments in a hot dry environment
Overall, the adsorption isotherms from the sediments from Coondiner Creek are classical “Type 1” curves, having an initial steep upward slope until saturation is exceeded and then reaches equilibrium as the sediment adsorption capacity is approached (Brunauer et al. 1938). Since Type 1 curves are typically monolayer, P and DOM appear to be primarily competing for limited surface adsorption sites in the sediment. While P adsorption behaviour of our sediments is comparable to lateritic soils of much finer texture (Singh and Gilkes 1991; Zhang et al. 2012), the sorption maxima (0.10–0.15 mg g−1) was much less than has been estimated in finer sediments high in Fe (Kerr et al. 2011) or indeed other tropical soils (0.7–5 mg g−1; Fontes and Weed 1996; Brenner et al. 2019). The specific surface area of iron oxides in the sediments used in this study (mostly 1–2 mm) is much less than sediments dominated by the clay and silt fractions that predominate in most sediments and which can account for a significant proportion of the variation in P adsorption among tropical soils (Fontes and Weed 1996; Brenner et al. 2019).
In our study, XRD and XRF analysis demonstrated that sediments were of a similar bulk chemical and mineralogical nature regardless of pool hydrology (Fig. 2). This consistency in sediment type across the 8 km of creekline examined here is likely due to a high degree of overall weathering of soils across the catchment as well as reworking during high discharge events, which homogenises the sediments at scales greater than individual pools. Streambed sediments in ephemeral dryland systems are typically fairly homogeneous compared to the fining of sediments with distance downstream seen in perennial systems (Singer & Michaelides 2014; Datry et al. 2017). Further, the frequent natural drying and rewetting of our sediments under field conditions along with prolonged exposure to high air temperatures (~ 45 °C)—such that temperatures of exposed soils and dry stream sediments exceed 60 °C throughout the day—will also have contributed to a consistent iron crystallinity of sediments among pools (Attygalla et al. 2016). We note that although air-drying is indeed a ‘standard’ pre-treatment in sorption studies, it can alter extractable P concentrations (Simpson et al. 2018; Wang et al. 2020) and hence this treatment may dampen the likelihood of any effect observed.
Over periods of years to decades, sediments of ephemeral streams have prolonged exposure to drying, with only occasional rewetting. As stream intermittency increases globally (Acuña et al. 2015), sediment processes in these streams may experience more regular or extended dry periods, followed by a rewetting flush. It would be of interest to explicitly test how sediment P sorption is affected by extended drying. Our results suggest that sediment P sorption kinetics may be indirectly impacted by stream intermittency, for example, extended inter-flow periods allow for the accumulation organic matter from terrestrial inputs that are more humic increases DOM loads, which in turn can impact on stream nutrient cycling and metabolism (e.g. Acuña et al. 2015; Sabater et al. 2016). As far as we are aware, no study has investigated post-rewetting ‘reset time’ or the effect of historical frequency of wet/dry cycles on sediment sorption kinetics in aquatic systems, although the issue was indirectly explored by Baldwin et al. (2000). Some evidence exists in the soil science literature (Bünemann et al. 2013; Khan et al. 2019), although the focus is generally on P desorption (release) rather than adsorption. The amount of P desorped from sediments increases with increasing duration of drying period prior to rewetting (Schonbrunner et al. 2012). Baldwin et al. (2000) highlighted that microbially driven SO4 reduction is an important process contributing to sediment nutrient dynamics, while wet–dry cycling could lead to changes in sediment microbial communities (Baldwin and Mitchell 2000). It would be particularly useful to investigate changes in sorption kinetics coupled with an assessment of microbially driven anaerobic reductive dissolution of the mineral phases. More extensive characterisation of a diversity of mineralogies coupled with drying and rewetting experiments are also needed to better assess the significance of changing sediment P sorption properties for nitrogen and phosphorus limitations in dryland streams (Larned et al. 2010).
Interactions between DOM and P adsorption
This study demonstrates that along with phosphorus, these stream sediments are a sink for more ‘recalcitrant’, humic-like DOM. The preferential removal from solution of DOM exhibiting high spectral slope (S275–295) when P was absent or at low experimental concentrations (< 10 mg L−1), coincides with P saturation for the stream sediments used in this study. While this study did not directly measure DOM molecular weights, molecular weight is strongly correlated to spectral slope (Helms et al. 2008; Wagner et al. 2015; Wunsch et al. 2018). Previous studies have also shown that high molecular weight DOM is preferentially sorbed to goethite (Ohno et al. 2007), a key mineral in our sediments. The analyses of decreased SUVA254 (Fig. 6), spectral slope (S275–295), humification index (HIX) (Fig. 7), and fluorescing humic-like components (Fig. 8) suggests that DOM retained in solution at lower P concentrations was of decreased aromaticity. Thus, it is likely that aromatic high molecular weight (HMW) DOM is being preferentially adsorbed on to sediments. Preferential adsorption may also help explain recent observations of retention of HMW fractions retained at the hyporheic interface of other streams (Harjung et al. 2018). In contrast, we would expect decreased aromaticity at higher initial P concentrations if this finding was a result of P bonding to DOM in solution and breaking conjugated bonds. Overall, our results are consistent with other studies that have investigated DOM adsorption in isolation from the effects of P, showing increased molecular weight of DOM remaining in solution with increasing DOM concentration (Gu et al. 1995; Meier et al. 1999).
Phosphorus addition enhanced desorption of native DOM from the Fe-rich sediments examined. Fluorescence analysis revealed that humic acids (components C1 and C2) were desorbed from sediments (Fig. 8), most notably when initial P concentration was high. However, this was not discernible from DOC analysis alone as DOC concentrations were below detection limit. This finding suggests that P replaces DOM associated with sediment surfaces, causing the release of stored DOM back to the water column. Increasing P concentrations have been shown to enhance the release of DOM from peat sediments (Sokolowska et al. 2011). In eutrophic systems, the presence of organic matter may also limit desorption of inorganic P through preferential desorption of dissolved organic P (Wang et al. 2008). Therefore, we suggest that assessment of sediment P sorption dynamics under field conditions should consider not only mineralogy and sediment composition but also the composition and dynamics of inputs of DOM, especially from humic-rich allochthonous sources.
The P dynamics in sub-tropical ephemeral streams are poorly understood, particularly the P sorption behaviours of sediments and the role of organic matter. For the iron-rich and highly weathered sediments of the catchment of our study stream, P sorption capacity was shown to be largely controlled by the nature of these sediments. While the iron-rich sediments can act as a sink for P, within the stream pools the concentration of DOM in the water column and in sediment pore water are highly likely to play a role in regulating P desorption. Furthermore, there are complex ways that DOM may interact with the iron-rich sediments to influence P availability. The concentrations of potentially biologically ‘available’ P in the in the pools and sediments, are to a large extent mediated by the P adsorption and desorption processes. These interactions, along with other processes such as microbial activity, are particularly important during the protracted periods of no flows that are characteristic of ephemeral stream systems.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Acuña V, Casellas M, Corcoll N, Timoner X, Sabater S (2015) Increasing extent of periods of no flow in intermittent waterways promotes heterotrophy. Freshw Biol 60:1810–1823. https://doi.org/10.1111/fwb.12612
Attygalla NW, Baldwin DS, Silvester E, Kappen P, Whitworth KL (2016) The severity of sediment desiccation affects the adsorption characteristics and speciation of phosphorus. Environ Sci Process Impacts 18:64–71. https://doi.org/10.1039/c5em00523j
Baken S, Moens C, van der Grift B, Smolders E (2016) Phosphate binding by natural iron-rich colloids in streams. Water Res 98:326–333. https://doi.org/10.1016/j.watres.2016.04.032
Baker A et al (2014) Chemometric analysis of organic matter fluorescence. Aquatic organic matter fluorescence. Cambridge environmental chemistry series. Cambridge University Press, Cambridge, pp 339–375. https://doi.org/10.1017/cbo9781139045452.016
Baldwin DS (1996) Effects of exposure to air and subsequent drying on the phosphate sorption characteristics of sediments from a eutrophic reservoir. Limnol Oceanogr 41:1725–1732. https://doi.org/10.4319/lo.1996.41.8.1725
Baldwin DS (1999) Dissolved organic matter and phosphorus leached from fresh and “terrestrially” aged river red gum leaves: implications for assessing river-floodplain interactions. Freshw Biol 41:675–685. https://doi.org/10.1046/j.1365-2427.1999.00404.x
Baldwin DS, Mitchell AM (2000) The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river–floodplain systems: a synthesis Regulated Rivers. Res Manag 16:457–467. https://doi.org/10.1002/1099-1646(200009/10)16:5%3c457::Aid-rrr597%3e3.0.Co;2-b
Baldwin DS, Mitchell AM, Rees GN (2000) The effects of in situ drying on sediment-phosphate interactions in sediments from an old wetland. Hydrobiologia 431:3–12. https://doi.org/10.1023/a:1004015019608
Beesley LS, Prince J (2010) Fish community structure in an intermittent river: the importance of environmental stability, landscape factors and within-pool habitat descriptors. Marine Freshw Res 61(5):605–614. https://doi.org/10.1071/MF09137
Bentley D, Grierson PF, Bennett LT, Adams MA (1999) Evaluation of anion exchange membranes to estimate bioavailable phosphorus in native grasslands of semi-arid northwestern Australia. Commun Soil Sci Plant Anal 30(15–16):2231–2244. https://doi.org/10.1080/00103629909370368
Bowman RA, Cole CV (1978) An exploratory method for fractionation of organic phosphorous from grassland soils. Soil Sci 125:95–101. https://doi.org/10.1097/00010694-197802000-00006
Brenner J, Porter W, Phillips JR, Childs J, Yang XJ, Mayes MA (2019) Phosphorus sorption on tropical soils with relevance to earth system model needs. Soil Res 57(1):17–27. https://doi.org/10.1071/Sr18197
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Bünemann EK, Keller B, Hoop D, Jud K, Boivin P, Frossard E (2013) Increased availability of phosphorus after drying and rewetting of a grassland soil: processes and plant use. Plant Soil 370(1–2):511–526. https://doi.org/10.1007/s11104-013-1651-y
Charles S, Fu G, Silberstein R, Mpelasoka F, McFarlane D, Hodgson G, Teng J, Gabrovsek C et al. (2015) Hydroclimate of the Pilbara: past, present and future. A technical report to the Government of Western Australian and industry partners from the CSIRO Pilbara Water Resource Assessment. Australia
Cheng WP, Chi FH, Yu RF (2004) Effect of phosphate on removal of humic substances by aluminum sulfate coagulant. J Colloid Interface Sci 272:153–157. https://doi.org/10.1016/j.jcis.2003.08.074
Datry T, Bonada N, Boulton AJ (2017) Intermittent rivers and ephemeral streams: ecology and management. Elsevier Science & Technology, San Diego
Datry T et al (2018) A global analysis of terrestrial plant litter dynamics in non-perennial waterways. Nat Geosci. https://doi.org/10.1038/s41561-018-0134-4
Daugherty EE, Gilbert B, Nico PS, Borch T (2017) Complexation and redox buffering of iron(II) by dissolved organic matter. Environ Sci Technol 51:11096–11104. https://doi.org/10.1021/acs.est.7b03152
Dogramaci S, Skrzypek G, Dodson W, Grierson PF (2012) Stable isotope and hydrochemical evolution of groundwater in the semi-arid Hamersley Basin of subtropical northwest Australia. J Hydrol 475:281–293. https://doi.org/10.1016/j.jhydrol.2012.10.004
Dogramaci S, Firmani G, Hedley P, Skrzypek G, Grierson PF (2015) Evaluating recharge to an ephemeral dryland stream using a hydraulic model and water, chloride and isotope mass balance. J Hydrol 521:520–532. https://doi.org/10.1016/j.jhydrol.2014.12.017
Fellman JB, Dogramaci S, Skrzypek G, Dodson W, Grierson PF (2011) Hydrologic control of dissolved organic matter biogeochemistry in pools of a subtropical dryland river. Water Resour Res. https://doi.org/10.1029/2010wr010275
Fellman JB, Petrone KC, Grierson PF (2013) Leaf litter age, chemical quality, and photodegradation control the fate of leachate dissolved organic matter in a dryland river. J Arid Environ 89:30–37. https://doi.org/10.1016/j.jaridenv.2012.10.011
Fink JR, Inda AV, Bavaresco J, Barrón V, Torrent J, Bayer C (2016) Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res 155:62–68. https://doi.org/10.1016/j.still.2015.07.017
Fontes MPF, Weed SB (1996) Phosphate adsorption by clays from Brazilian Oxisols: relationships with specific surface area and mineralogy. Geoderma 72(1–2):37–51. https://doi.org/10.1016/0016-7061(96)00010-9
Freundlich H (1907) Über die Adsorption in Lösungen. Z Phys Chem. https://doi.org/10.1515/zpch-1907-5723
Gonçalves JF, de Souza RR, Gregório RS, Valentin GC (2014) Relationship between dynamics of Litterfall and Riparian Plant species in a tropical stream limnologica. Ecol Manag Inland Waters 44:40–48. https://doi.org/10.1016/j.limno.2013.05.010
Grierson PF, Comerford NB, Jokela EJ (1998) Phosphorus mineralization kinetics and response of microbial phosphorus to drying and rewetting in a Florida Spodosol. Soil Biol Biochem 30:1323–1331. https://doi.org/10.1016/s0038-0717(98)00002-9
Gu B, Schmitt J, Chen Z, Liang L, McCarthy JF (1995) Adsorption and desorption of different organic matter fractions on iron oxide. Geochim Cosmochim Acta 59:219–229. https://doi.org/10.1016/0016-7037(94)00282-Q
Harjung A, Sabater F, Butturini A (2018) Hydrological connectivity drives dissolved organic matter processing in an intermittent stream. Limnologica 68:71–81. https://doi.org/10.1016/j.limno.2017.02.007
Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53:955–969. https://doi.org/10.4319/lo.2008.53.3.0955
Howitt JA, Baldwin DS, Rees GN, Hart BT (2008) Photodegradation, interaction with iron oxides and bioavailability of dissolved organic matter from forested floodplain sources. Marine Freshw Res 59:780–791. https://doi.org/10.1071/mf07225
Iles JA (2019) Nutrient limitation and interactions with organic matter and sediments within dryland streams of the Pilbara region of northwest Australia. Ph.D. Thesis, The University of Western Australia. https://doi.org/10.26182/5cd234d9b687d
Kennard MJ, Pusey BJ, Olden JD, Mackay SJ, Stein JL, Marsh N (2010) Classification of natural flow regimes in Australia to support environmental flow management. Freshw Biol 55(1):171–193. https://doi.org/10.1111/j.1365-2427.2009.02307.x
Kerr JG, Burford M, Olley J, Udy J (2010) The effects of drying on phosphorus sorption and speciation in subtropical river sediments. Mar Freshw Res 61:928–935. https://doi.org/10.1071/MF09124
Kerr J, Burford M, Olley J, Udy J (2011) Phosphorus sorption in soils and sediments: implications for phosphate supply to a subtropical river in southeast Queensland, Australia. Biogeochemistry 102:73–85. https://doi.org/10.1007/s10533-010-9422-9
Khan SU, Hooda PS, Blackwell MSA, Busquets R (2019) Microbial biomass responses to soil drying-rewetting and phosphorus leaching. Front Environ Sci. https://doi.org/10.3389/fenvs.2019.00133
Knowles L, Iles J, Lu Y, Kobayashi T, Wen L (2012) Phosphorus dynamics in an ephemeral wetland ecosystem after re-flooding. Environ Model Softw 35:31–37. https://doi.org/10.1016/j.envsoft.2012.02.003
Kuo S (1996) Phosphorus. In: Sparks DL (ed) Methods of soil analysis: part 3. SSSA Book Series No. 5. SSSA and ASA, Madison, Madison, pp 869–919
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Larned ST, Datry T, Arscott DB, Tockner K (2010) Emerging concepts in temporary-river ecology. Freshw Biol 55(4):717–738. https://doi.org/10.1111/j.1365-2427.2009.02322.x
McDowell RW, Sharpley AN (2001) A comparison of fluvial sediment phosphorus (P) chemistry in relation to location and potential to influence stream P concentrations. Aquat Geochem 7:255–265. https://doi.org/10.1023/a:1015274426411
Meier M, Namjesnik-Dejanovic K, Maurice PA, Chin YP, Aiken GR (1999) Fractionation of aquatic natural organic matter upon sorption to goethite and kaolinite. Chem Geol 157:275–284. https://doi.org/10.1016/S0009-2541(99)00006-6
Mitchell A, Baldwin DS (1998) Effects of desiccation/oxidation on the potential for bacterially mediated P release from sediments. Limnol Oceanogr 43:481–487. https://doi.org/10.4319/lo.1998.43.3.0481
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Murphy KR, Stedmon CA, Graeber D, Bro R (2013) Fluorescence spectroscopy and multi-way techniques. PARAFAC Anal Methods. https://doi.org/10.1039/c3ay41160e
Murphy KR, Stedmon CA, Wenig P, Bro R (2014) OpenFluor—an online spectral library of auto-fluorescence by organic compounds in the environment. Anal Methods 6:658–661. https://doi.org/10.1039/c3ay41935e
Nair PS, Reddy KR (2013) Phosphorus sorption and desorption in wetland soils. In: DeLaune RD, Reddy KR, Richardson CJ, Megonigal JP (eds) Methods in biogeochemistry of wetlands, vol 10. Soil Science Society of America, New York. https://doi.org/10.2136/sssabookser10.c34
Ohno T (2002) Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ Sci Technol 36:742–746. https://doi.org/10.1021/es0155276
Ohno T, Chorover J, Omoike A, Hunt J (2007) Molecular weight and humification index as predictors of adsorption for plant- and manure-derived dissolved organic matter to goethite. Eur J Soil Sci 58:125–132. https://doi.org/10.1111/j.1365-2389.2006.00817.x
Pant HK, Reddy KR (2001) Phosphorus sorption characteristics of estuarine sediments under different redox conditions. J Environ Qual 30:1474–1480. https://doi.org/10.2134/jeq2001.3041474x
R Core Team (2017) R: a language and environment for statistical computing R Foundation for Statistical Computing. Austria, Vienna
Ramanaidou ER, Morris RC, Horwitz RC (2003) Channel iron deposits of the Hamersley Province, Western Australia. Aust J Earth Sci 50:669–690. https://doi.org/10.1111/j.1440-0952.2003.01019.x
Reddy KR, Kadlec RH, Flaig E, Gale PM (1999) Phosphorus retention in streams and wetlands: a review. Crit Rev Environ Sci Technol 29:83–146. https://doi.org/10.1080/10643389991259182
Rouillard A, Skrzypek G, Dogramaci S, Turney C, Grierson PF (2015) Impacts of high inter-annual variability of rainfall on a century of extreme hydrologic regime of northwest Australia. Hydrol Earth Syst Sci 19:2057–2078. https://doi.org/10.5194/hess-19-2057-2015
Sabater S, Timoner X, Borrego C, Acuña V (2016) Stream biofilm responses to flow intermittency: from cells to ecosystems. Front Environ Sci. https://doi.org/10.3389/fenvs.2016.00014
Schonbrunner IM, Preiner S, Hein T (2012) Impact of drying and re-flooding of sediment on phosphorus dynamics of river-floodplain systems. Sci Total Environ 432:329–337. https://doi.org/10.1016/j.scitotenv.2012.06.025
Shutova Y, Baker A, Bridgeman J, Henderson RK (2014) Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: from PARAFAC analysis to online monitoring wavelengths. Water Res 54:159–169. https://doi.org/10.1016/j.watres.2014.01.053
Siebers AR, Pettit NE, Skrzypek G, Fellman JB, Dogramaci S, Grierson PF (2016) Alluvial ground water influences dissolved organic matter biogeochemistry of pools within intermittent dryland streams. Freshw Biol 61:1228–1241. https://doi.org/10.1111/fwb.12656
Siebers AR, Pettit NE, Skrzypek G, Dogramaci S, Grierson PF (2020) Diel cycles of δ13CDIC and ecosystem metabolism in ephemeral dryland streams. Aquatic Sci 82(2):32. https://doi.org/10.1007/s00027-020-0708-2
Siebers AR (2015) Organic matter dynamics of hydrologically variable pools in intermittent streams of northwest Australia. Ph.D. Thesis, The University of Western Australia
Simpson ZP, McDowell RW, Condron LM (2018) The error in stream sediment phosphorus fractionation and sorption properties effected by drying pretreatments. J Soils Sediments 19(3):1587–1597. https://doi.org/10.1007/s11368-018-2180-3
Singer MB, Michaelides K (2014) How is topographic simplicity maintained in ephemeral dryland channels? Geology 42(12):1091–1094. https://doi.org/10.1130/g36267.1
Singh B, Gilkes R (1991) Phosphorus sorption in relation to soil properties for the major soil types of south-western Australia. Soil Res 29:603–618. https://doi.org/10.1071/SR9910603
Sokolowska Z, Szajdak L, Boguta P (2011) Effect of phosphates on dissolved organic matter release from peat-muck soils. Int Agrophys 25:173–180
Song K-Y, Zoh K-D, Kang H (2007) Release of phosphate in a wetland by changes in hydrological regime. Sci Total Environ 380:13–18. https://doi.org/10.1016/j.scitotenv.2006.11.035
Stedmon CA, Bro R (2008) Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Limnol Oceanogr Methods 6:572–579. https://doi.org/10.4319/lom.2008.6.572
Tonin AM et al (2017) Plant litter dynamics in the forest-stream interface: precipitation is a major control across tropical biomes. Sci Rep 7:10799. https://doi.org/10.1038/s41598-017-10576-8
Turner A, Rawling MC (2001) The influence of salting out on the sorption of neutral organic compounds in estuaries. Water Res 35(18):4379–4389. https://doi.org/10.1016/s0043-1354(01)00163-4
Verbeeck M, Hiemstra T, Thiry Y, Smolders E (2017) Soil organic matter reduces the sorption of arsenate and phosphate: a soil profile study and geochemical modelling. Eur J Soil Sci 68:678–688. https://doi.org/10.1111/ejss.12447
Wagner S, Jaffé R, Cawley K, Dittmar T, Stubbins A (2015) Associations between the molecular and optical properties of dissolved organic matter in the Florida everglades, a model coastal wetland system. Front Chem 3:66–66. https://doi.org/10.3389/fchem.2015.00066
Wang SR, Jin XC, Zhao HC, Zhou XN, Wu FC (2008) Effects of organic matter on phosphorus release kinetics in different trophic lake sediments and application of transition state theory. J Environ Manage 88:845–852. https://doi.org/10.1016/j.jenvman.2007.04.006
Wang J, Wu Y, Zhou J, Bing H, Sun H, Luo J, Pu S (2020) Air-drying changes the distribution of Hedley phosphorus pools in forest soils. Pedosphere 30(2):272–284. https://doi.org/10.1016/s1002-0160(17)60456-9
Watts CJ (2000a) The effect of organic matter on sedimentary phosphorus release in an Australian reservoir. Hydrobiologia 431:13–25. https://doi.org/10.1023/a:1004046103679
Watts CJ (2000b) Seasonal phosphorus release from exposed, re-inundated littoral sediments of two Australian reservoirs. Hydrobiologia 431:27–39. https://doi.org/10.1023/a:1004098120517
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708. https://doi.org/10.1021/es030360x
Wunsch UJ, Stedmon CA, Tranvik LJ, Guillemette F (2018) Unraveling the size-dependent optical properties of dissolved organic matter. Limnol Oceanogr 63:588–601. https://doi.org/10.1002/lno.10651
Yan JL, Jiang T, Yao Y, Wang J, Cai YL, Green NW, Wei SQ (2017) Underestimation of phosphorus fraction change in the supernatant after phosphorus adsorption onto iron oxides and iron oxide-natural organic matter complexes. J Environ Sci 55:197–205. https://doi.org/10.1016/j.jes.2016.08.005
Zhang L, Hong S, He J, Gan F, Ho Y-S (2012) Adsorption characteristic studies of phosphorus onto laterite. Desalin Water Treat 25:98–105. https://doi.org/10.5004/dwt.2011.1871
Acknowledgements
We acknowledge the Traditional Owners, the Nyiyaparli, of the lands which this study was conducted, and would also like to pay our respects to Elders past and present. Douglas Ford and Andre Siebers assisted with field sample collection. We thank Naoko Zwingmann and Shawan Dogramaci (RTIO) for providing geochemical analysis of sediment samples. We would like to thank the anonymous reviewers for the valuable comments and suggestions which helped us to improve the quality of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Financial and logistic contributions to fieldwork and laboratory expenses were provided to PFG by ARC linkage grant LP120200002 in collaboration with Rio Tinto (RTIO) and to JAI by Pilbara Corridors Biodiversity Scholarship from Rangelands NRM Western Australia. The authors have no competing interests to declare that are relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iles, J.A., Pettit, N.E., Donn, M.J. et al. Phosphorus sorption characteristics and interactions with leaf litter-derived dissolved organic matter leachate in iron-rich sediments of a sub-tropical ephemeral stream. Aquat Sci 84, 56 (2022). https://doi.org/10.1007/s00027-022-00888-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00888-x