Abstract
We described the spatial and temporal dynamics of aquatic macrophytes in a Neotropical coastal estuarine river, and identified the negative effects associated to the presence and dominance of the invasive tanner grass. We compared macrophyte beds along the Guaraguaçu River (South Brazil) over four years, using taxonomic and functional dimensions. Biodiversity descriptors were higher in the driest periods compared to the rainiest, although this difference seems to be decreasing over the studied years. Moreover, the spatial organization of biodiversity and community structure slightly changed over time. Such spatial community stability was highlighted by the dominance of the highly invasive tanner grass. In beds dominated by the invasive species, the biodiversity was reduced. As aquatic macrophytes represent an important group in water bodies, the long-term loss of seasonal differences in community structuring is of concern, mainly regarding its potential to impact other groups and ecosystem functioning. By analysing data from standardized monitoring, we were able to identify a poorly discussed facet of biotic homogenisation—the seasonal homogenisation. We also discussed the impact of massive development of invasive species and its consequences for biodiversity in a Neotropical river of outmost importance for biological conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A myriad of interactive ecological factors contributes to explain biodiversity in ecosystems. It is well established for aquatic macrophytes that spatial and temporal changes in species composition are explained by a combination of processes, including seasonal changes, dispersion, hydrological variation and environmental filtering (Lacoul and Freedman 2006; Bornette and Puijalon 2011; Fritz et al. 2017; Ceschin et al. 2017). At larger scales, dispersion and landscape (including hydrology) are common drivers of the diversity of aquatic plants (Rolon et al. 2010). Within an ecosystem without barriers for dispersal, environmental filtering of species is the predominant factor. Characteristics such as nutrient concentrations (Lacoul and Freedman 2006) and salinity are important drives of macrophyte communities (Alahuhta et al. 2020) and, in this sense, precipitation is expected to be a key factor shaping macrophyte communities (Pavão et al. 2017), given it can directly control nutrient load, physico-chemical characteristics, and also affect species’ dispersion particularly in environments with seasonal differences in rainfall and with geographic features that highlight effects of rain in hydrology.
At long term, there is a growing consensus that anthropogenic changes in the abiotic and biotic characteristics of a system is the most important underlying cause for species distributions (Pozo et al. 2011; Elo et al. 2018). Among the consequences of anthropogenic impacts is the promotion of invasive species. Negative impacts associated with invasive species are well described (Simberloff et al. 2013), particularly in aquatic systems (Thomaz et al. 2015). In this respect, the dominance of invasive macrophyte species is probably the most emblematic anthropogenic impact on aquatic macrophyte communities at ecosystem scale (Havel et al. 2015; Bolpagni et al. 2020). To illustrate the importance of this impact, the Brazilian hyacinth Pontederia crassipes Mart. is considered one of the world’s worst invasive species (Lowe et al. 2000). Interestingly, in some Brazilian aquatic environments in which P. crassipes is native, one of the most successful invasive species is the African tropical tanner grass Urochloa arrecta (Hack. ex T. Durand & Schinz) Morrone & Zuloaga (see Amorim et al. 2015). This species was introduced in many countries as a robust and resilient pasture grass, a former common practice in coastal areas of South Brazil (Araújo et al. 2021). In Brazil, the sad history of Urochloa species invasion is also associated to accidental introduction given its use as mattress for slaves in slave ships (Sato et al. 2021). The high growth rate, wide physiological tolerance and rapid evolutionary responses of this species result in a very aggressive invader in aquatic macrophyte communities (Michelan et al. 2010; Bora et al. 2020). It is well documented that U. arrecta dominates aquatic macrophyte beds and replaces native plant species (Amorim et al. 2015). Nonetheless, this response may depend on the richness and biomass of the native species (Michelan et al. 2013). Therefore, one can expect that different facets of biodiversity will be distinctly affected by the occurrence and dominance of U. arrecta in macrophyte beds.
Biodiversity is increasingly being assessed using a functional approach in addition to the usual taxonomy-based ecological indices, such as species richness and diversity (Vecchia et al. 2020). Indeed, it has been recognized that functional diversity might help to understand the relationship between the biota and ecosystem functioning, as well as the effects of disturbances on the ecosystem’s stability and resiliency (Petchey and Gaston 2006; Cadotte et al. 2011; Flynn et al. 2011). Similarly, functional diversity may also be a better proxy for the effects of anthropogenic drivers on the phenomenon of biotic homogenisation (or ‘biotic homogenization’, see Olden et al. 2018). Management actions that could serve to maintain or even increase diversity over time and space are better defined if the functional role of species in ecosystems is taken into consideration (Song et al. 2014). In this sense, it is important to highlight the need for long-term monitoring programs in order to capture the major temporal and spatial patterns occurring in the different components of biodiversity (Hussner 2014); particularly given that nuisance control of macrophytes is extremely difficult (Hussner et al. 2017). Beyond that, long-term monitoring can help to detect effects of environmental changes, and can provide evidences of biotic homogenisation as a result of species invasions (Strayer et al. 2006).
Here, we described the spatial and temporal dynamics of aquatic macrophyte diversity (both taxonomic and functional) in the Guaraguaçu River, a subtropical coastal tidal river in southern Brazil that has been highly invaded by U. arrecta (Araújo et al. 2021). We hypothesized that the main drivers of macrophyte diversity are: i) seasonal pattern of precipitation driving the temporal variation, and ii) the presence and dominance of the invasive tropical tanner grass and, the changes in environmental conditions along the river, leading to spatial variation. Yet, regarding the spatial variation, we expected lower values of taxonomic and functional diversity associated to increased U. arrecta dominance, and in sites near the mouth of the river, given that salinity is a limiting factor for aquatic plant colonization (Haller et al. 1974).
Materials and methods
Study area
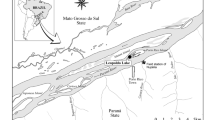
The Guaraguaçu River (25° 37′ 36.4″ S, 48° 30′ 29.2″ W, Fig. 1) is a tropical river with a strong tidal influence (it discharges into the Paranaguá Bay) and strong salinity gradient (in the last sampling, the salinity ranged from 53.1 to 7460 µS/cm in the first and last sites sampled, respectively). Also, there is conspicuous longitudinal variation, with distinct microhabitats along the course of the river (Lana et al. 2001). It is colonized by aquatic macrophytes along its entire length. The river has a linear total extent of about 60 km and is the largest river of the coastal basin of Paraná State, South Brazil. It is located in one of the best preserved reminiscent of the Atlantic Forest biodiversity hotspot (Myers et al. 2000), known as Lagamar. Lagamar is the set of estuaries in South-Southeast Brazil (mainly Paraná and São Paulo States) with extremely high importance for conservation, considered as a Biosphere Reserve and World Heritage Site (<https://whc.unesco.org/en/list/893/>). Its headwaters include multiple streams located in the Serra da Prata/Serra do Mar range at an elevation of 766 m above sea level, inside the Saint-Hilaire/Hugo Lange National Park. The region has a tropical and super-humid climate. The average accumulated rainfall is 2300 mm year−1. The rainfall is distributed throughout the year, but there are marked differences between summer and winter: the rainiest season occurs in summer, with an accumulation of about 800 mm year−1, and the driest season occurs in winter, with an accumulation of about 280 mm year−1 (Vanhoni and Mendonça 2008). Given the geographic layout of Guaraguaçu river (formed by mountain streams and flowing in the coastal plain), water flow and material leaching is severely affected by extreme rainfall events that occur mainly during the rainiest season in the mountain chains that harbours the headwater streams (Pinto et al. 2012). The mean temperature is between 17 and 21 °C (Vitule et al. 2006).
Considering the changes in environmental characteristics and the nature of anthropic pressures along the river, there are three clearly distinct zones: the upstream near-pristine region known as “caixetal” [i.e., an ecosystem characterized by the high abundance of the amphibious endangered tree Tabebuia cassinoides Lam. (DC.) (Bignoniaceae)] (Araújo et al. 2021); the intermediate zone marked by the strong degradation in water quality associated with anthropic occupation (e.g., wastewater discharge); and the downstream brackish waters marked by less environmental degradation. This gradient has been shown to be important in the determining the structure of the fish association (Contente et al. 2011a).
In the intermediate region, there are two rectified channels used for public water supply, besides the mentioned reception of domestic wastewater. In this region, macrophyte species occur that are considered potentially invasive, such as U. arrecta (Fig. S1 in Appendix) and P. crassipes Mart. (even though native, P. crassipes can achieve invasive status in Brazilian waters, see Michelan et al. 2013; Pavão et al. 2017). The U. arrecta beds in this part of the river can reach hundreds of meters long, and dozens of meters large (A.A.P personal observation, see also Fig. S1 in Appendix); in contrast to its small occupation in sites nearby. In the upstream portion of the river, this invasive species is absent, as well as in most part of the mangrove at the river mouth (see Bora et al. 2020).
In addition, there are records of several non-native fish and invertebrate species, such as Clarias gariepinus (Burchell, 1822), Ictalurus punctatus (Rafinesque, 1818), Salminus brasiliensis (Cuvier, 1816), tilapias and Malaysian giant shrimp (Faria et al. 2021).
Data sampling
Sixteen macrophyte beds were selected and georeferenced along the navigable stretch of the river (Fig. 1). Samplings followed a standardized protocol for continuous monitoring (see details and metadata in <https://lasbufprbio.wixsite.com/home>) and occurred twice a year—in April, ending summer; and in September, ending winter—from 2016 to 2019. Unfortunately, data was not available for 2020 given samplings were cancelled after Sars-COV-2 pandemic. Taxonomic classification and trait measurements were performed in all sampling efforts. April and September were the chosen sampling months given the possible accumulative effects of summer and winter seasons on community structuring. Summer and winter are extremes not only in temperature, but also in precipitation and nutrient input in Guaraguaçu River. Precipitation in summer is substantially higher (Lana et al. 2001) as well as the input of domestic sewage in tributaries of the Guaraguaçu River given the high human density in coastal and touristic cities in the shoreline (IPARDES 2013).
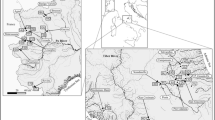
We followed the conventional methods of collection and herborization of aquatic macrophytes (Pedrali 1990) in all macrophyte beds. Specimens of each species in the reproductive stage were collected, herborized, and indexed in the scientific collection of the Botany Department Herbarium of the Federal University of Paraná (UPCB-UFPR). The minimum extent of each macrophyte bed selected was 50 m, so all macrophyte species within the georeferenced 50-m stretch were recorded—an adaptation for aquatic macrophytes of the RAPELD protocol (see Magnusson et al. 2005). The distances between macrophytes beds were never lower than 1 km. We estimated the abundance of each species recorded in macrophyte bed using Braun-Blanquet abundance classes, considering the occupancy of each species in the 50-m extension of macrophyte beds (0 = 0%; 1 = less than 5%; 2 = 5–25%; 3 = 25–50%; 4 = 50–75%; 5 = greater than 75% of occupancy).
Aquatic macrophytes are modular organisms with common vegetative reproduction. So, three modules (we consider a module a repeated part of the plant containing all vegetative organs) of each species recorded were selected from each macrophyte bed to measure the following functional traits: root length (cm), leaf length (cm), leaf width (cm), leaf area (cm2), petiole length (cm), stem width (cm) and herbivory degree (using the same Braun-Blanquet classes described above when considering the percentage of leaf area showing herbivory). Averages of each trait from all samplings were then used to calculate functional diversity (see ‘Data analysis’). We selected the most different modules possible in each macrophyte bed (the largest, the smallest and one common-sized intermediate) in an attempt to represent most trait variation of a species within a macrophyte bed. However, due to clonal and vegetative reproduction, it is very likely that modules of a same bed are from a same clonal individual. We consulted specialized bibliographies (APG 2009; Pott and Pott 2000; Moura-Junior et al. 2015) to determine the life form (emergent, rooted floating, free floating, rooted submerged, free submerged, amphibious, epiphyte) for each species. Functional traits abovementioned are commonly used in studies of aquatic macrophytes (e.g. Fu et al. 2014) and provide important information on biological responses to environmental variation, since they might affect critical stages of colonization and establishment, as well as the adaptive value of species (Violle et al. 2007; Cavalli et al. 2014).
Because of the close association of U. arrecta with degraded habitats (Araújo et al. 2021), we considered its presence to be an indicator of environmental quality. Therefore, we separated the aquatic macrophyte beds into three classes based on the abundance of U. arrecta: “Absent”, “Dominant” and “Not dominant”. A fourth class was used to designate the downstream Mangrove zone, where U. arrecta poorly occur, probably due the higher salinity (Bora et al. 2020).
Data analysis
We calculated the popular indices of local taxonomic (species richness, Pielou’s evenness and Shannon–Wiener) and functional (functional richness—FRic, functional divergence—FDiv, functional dispersion—FDis, functional evenness—FEve and Rao’s quadratic entropy—RaoQ; Laliberté and Legendre 2010) diversity (hereafter alpha diversity) for each sampling site in each sampling. The functional diversity indices were calculated using averages of all measurements of the traits. To assess the effect of seasonal rainfall on taxonomic and functional indices as evidence of a seasonal pattern, we ran a linear model, with permutations when the residuals were not normal. The mean diversity index was the response variable and the cumulative rainfall for the 15 days previous the start of field sampling was the explanatory variable indicative of seasonal effects. To calculate the cumulative rainfall, daily rainfall data was summed until the date of the sampling. These data were collected from the National Institute of Meteorology website <https://mapas.inmet.gov.br/>; the meteorological station is approximately 8 km from the Guaraguaçu River in straight line. The accumulated rainfall was also contrasted according to the month of sampling and according to the season (i.e., summer and winter) by performing an ANOVA.
The functional alpha diversity indices were compared among samplings using tests that account for repeated measures of the sites. In cases where a site or sample did not contain the minimum number of species necessary to calculate a particular diversity index, the index was not calculated. Incomplete data and the normality of its distribution were conditions driving the statistical analysis performed for each index: FRic, FEve and FDiv—Durbin Test, post hoc Conover Iman Test; FDis—linear mixed effects model, post hoc Tukey Test; RaoQ—Friedman Test, post hoc Conover Iman Test.
We then assessed the spatial variation along the river by contrasting the number of shared species pairwise with the distance among the respective sites, using an ordinary linear model or a linear model with permutations when the residuals were not normal. The dependent variable was the number of shared species and the independent variable was the distance. The distance was manually calculated by connecting the sites in QGis software version 3.10.4 (QGIS.org 2021).
The number of species at each site shared with the other sites was evaluated in relation to accumulated rainfall and between the samplings. In the first case, we used the sum of shared species in the sampling period as the dependent variable and the rainfall as the independent variable. In the second case, we used the Friedman test, considering the pairwise differences as the blocks. Post hoc pairwise comparisons were performed by Conover–Iman test.
Temporal changes in community composition were assessed using two approaches. First, we used the temporal beta diversity index (TBI; Legendre 2019). In TBI, a dissimilarity index is calculated for the same sites between two moments or times, and through permutations the sites which had an extraordinary level of dissimilarity in relation to the others are identified. The analysis can also provide the components loss (B) and gain (C) of the dissimilarity index, and they can be differentiated through a permuted paired t test. Therefore, the analysis can inform us if there were more losses or gains in abundance-per-species and/or species occurrences between the distinct times or dates. The second approach was the betadisper function (Anderson et al. 2006), intended to inform about the whole dissimilarity (using a Bray–Curtis dissimilarity index) of the river compared between each sampling. In summary, the betadisper approach calculates the dispersion around the centroid of a given group (i.e. variance) using a dissimilarity matrix, and then compares the dispersion among groups. In our case, the betadisper approach informed us if there was any sampling period with higher or lower taxonomic variation among the sites sampled in the river. Also, compositional changes were evaluated through Permutational Multivariate Analysis of Variance (PERMANOVA; Anderson 2001). The TBI and betadisper approaches were also performed using the community-level weighted means of trait values (CWM), in order to analyse temporal changes in traits. For that, the Euclidean distances were applied to standardized CWM values. The CWM is the average value of a trait in the community, considering the relative abundances of each species. Therefore, CWM represents the dominant value of a given trait in the community.
We used the categories of dominance of U. arrecta to evaluate its possible effects on the functional and taxonomic indexes. Then, we compared the indices from each dominance category in each sampling using ANOVA. Betadisper was used with the dominance categories as the groups for comparisons of the homogeneity of variances, both with the taxonomic and CWM matrices. We also performed a comparison of the dominance categories using data from all samplings. In this case, we performed a linear mixed effects model (distances to centroid ~ category of dominance, random = ~ 1|sampling) and used Tukey Test for post hoc pairwise comparisons. Finally, we used the analysis of Indicator Value (IndVal, Dufrene and Legendre 1997) to evaluate if and which species were typical for each class of U. arrecta dominance. We called the species significantly associated with the classes as the indicator species. We used these analyses as well to explore indicator species of sampling sites and periods (summer and winter, see Table S1 in Appendix).
All analyses and graphing were performed in R software, version 4.0.2 (R Core Team 2020; see Table S1 in Appendix to specific functions and packages used). For all tests, we considered significant differences when type I error was lower than 5%.
Results
The accumulated rainfall differed between summer (mean 998.25 ± 305.03 mm) and winter (mean 159.42 ± 135.86 mm; F1,6 = 25.24, P = 0.00239; Fig. 2a). However, the accumulated rainfall of the 15 days before the sampling was not different between April (mean 103.9 ± 72.95 mm) and September (mean 30.7 ± 34.49 mm; F1,6 = 3.291, P = 0.12; Fig. 2b).
A total of 39 species were identified. According to the species accumulation curve (Fig. S2 in Appendix), these were a good representation of the aquatic macrophyte community of the Guaraguaçu River. The maximum number of species sampled in one macrophyte bed was 20, in September 2018 at sampling site 11. The minimum of one species was recorded in site 16 (i.e., the last mangrove site) in five samplings (April 2017 and September of all years), and at sites 15 and 9 at September 2016. The minimum and maximum values of Shannon–Wiener index were 0.60 and 2.85. The maximum was associated with the same site and sampling period where the maximum richness was found; the minimum value was at sampling site 1 in September 2016. The highest Pielou index value was recorded at site 10 in April 2017, and the lowest non-zero value was recorded at site 16 in September 2016. The site 15 in April 2016 and September 2017 reached the value of 1 for Pielou, but as it was a bias of the reduced number of species, we did not consider it as the maximum.
Spatial and temporal patterns in alpha diversity
There was no relationship between the 15 days accumulated rainfall before the samplings and the alpha taxonomic species richness, averaged Shannon–Wiener index and averaged Pielou index in any of the samplings (Fig. S3 in Appendix).
The sum of shared species among pairs of sites was not dependent on the rainfall accumulated 15 days before the samplings (Fig. 3), although more species were shared in summer than in winter in 2018 and 2019 (chi-squared = 307.52, P < 0.001; Fig. 4). On the other hand, the number of shared species in pairs of sites was dependent on the distance between sites in all periods (see Fig. S4 in Appendix), confirming that more species are shared between nearby sites. There is clear spatial autocorrelation consistent to river-continuous dependency.
Boxplots of the number of shared species pairwise of each site and in each sampling. The lines represent the temporal evolution of each site. Intensity in the color of lines represent overlap of data, i.e., sites with the same number of shared species. Different letters on the top represent differences in median among the samplings, obtained by the Conover–Iman test after running Friedman test. The red line in the boxes represent the median, and the limits of the box the first and third quartiles. The whiskers represent the lowest and highest values in the range determined by 1.5 times the difference between the interquartiles, and the points are outliers. April/16–April/19 indicate April 2016–April 2019; and Sep/16–Sep/19 indicate September 2016–September 2019
Similar to the results regarding the taxonomic indexes, the averaged functional alpha diversity indices were not related to the accumulated rainfall during the 15 days previously the samplings (Fig. S5 in Appendix). However, Fric, Fdis and RaoQ entropy differed between the samplings. In general, there was a trend of higher values in April in comparison to September, and this pattern of increase in April and decrease in September was more consistent in Fric (Fig. 5 and Fig. S6 in Appendix). In relation to the spatial distribution of functional diversity indices, elevated values were, in general, associated with sites 5 and 6 (Fig. S7 in Appendix).
Boxplots of functional richness index calculated in each sampling. Different letters indicate differences among the samplings, obtained by the Conover–Iman test after performing Durbin Test (chi-square = 67.562, P < 0.001). April/16–April/19 indicate April 2016–April 2019; and Sep/16–Sep/19 indicate September 2016–September 2019. The limits of the box represent the first and the third quartiles. The line inside the box is the median, and the whiskers represent the lowest and highest values in the range determined by 1.5 times the difference between the interquartiles. Points represent outliers
The temporal beta diversity is shown in Fig. 6, both as abundance-per-species and occurrence data. Sites that changed significantly between the samplings were only detected using the occurrence data, in the comparisons April/18-Sep/18—site 15, Sep/18-April/19—site 16 and April/19-Sep/19—site 16. Yet, on some occasions, the gain and loss components of TBI differed, and gains in abundance-per-species and number of species were associated with transition from winter to summer (Table 1 and asterisks in Fig. 6). Irrespective of the type of the data, abundance-per-species or occurrence, a trend toward a reduction in the mean dissimilarity can be observed. Thus, during the period studied, the sites reduced the seasonal variability between summer and winter.
Mean dissimilarity (i.e., TBI) over time (comparisons between consecutive samplings). Distinct colors represent the TBI and its components loss (B) and gain (C). Standard deviation of mean dissimilarity is shown. Asterisks indicate when loss and gain components were different, after permuted paired T-test. April/16–April/19 indicate April 2016–April 2019; and Sep/16–Sep/19 indicate September 2016–September 2019
No differences in the distances to the centroids among the sampling periods using betadisper approach were found (PERMUTEST: F7,120 = 0.5655; P = 0.7913; Fig. 7a, c); meaning that the dissimilarities in community composition in the river were the same during the studied period. Also, no significant changes in species composition occurred (PERMANOVA: F7,120 = 1.1068; P = 0.2943).
Top: compositional (a) and functional (b) dispersion of each period. The central circles are the centroids. Distinct colors and symbols indicate the year and month of samplings, respectively. Bottom: mean ± standard deviation of distances to centroid in each sampling, taxonomic (c) and functional (d). April/16–April/19 indicate April 2016–April 2019; and Sep/16–Sep/19 indicate September 2016–September 2019
The analysis of temporal functional beta diversity, i.e., application of the TBI approach on the CWM matrix, revealed the same pattern as the taxonomic data: a reduction in the mean dissimilarity between consecutive seasons experienced by the sites over the four-years studied period (Fig. 8). Variations in the B (reduction) and C (increase) of traits’ values were not significant; only at sites 9 (April/16–Sep/16) and 16 (April/19–Sep/19) was the dissimilarity exceptionally higher than at the other sites. In all comparisons (except April/19–Sep/19), at least one trait varied significantly (Table S2 in Appendix). The two clearest patterns of increase and reduction over time are associated with leaf length and leaf width, in opposite directions. In winter, the length of the leaves usually increased and the width decreased. The stem width increased in winter and foliar area in summer, but this was not as consistent as the leaf patterns. Regarding the variability in traits among sites, the variances were the same in all samplings (PERMUTEST: F7,120 = 0.8794, P = 0.5242, Fig. 7b, d).
Temporal mean functional TBI calculated from CWM matrix data. The bars indicate standard deviation. No differences between the increase and decrease components of TBI were found by permuted paired t-test. April/16–April/19 indicate April 2016–April 2019; and Sep/16–Sep/19 indicate September 2016–September 2019
Effects of the invasive tanner grass
In none of the analyses, the abundance of U. arrecta was related to either 15 days of accumulated rainfall before sampling or sampling period. It is important to mention that almost all sites where U. arrecta was absent are located in the upper area of the river, the ‘Caixetal’ ecosystem, and in monitored sites of the mangrove area. Therefore, the distribution of this species along the river is between the sites 5 and 14.
Changes in alpha taxonomic indexes were found when contrasting the U. arrecta dominance categories and the mangrove. In most cases, the difference was between the Mangrove and the categories of dominance of U. arrecta (see Table S3 in Appendix for averages and standard deviations).
Analysing the relation of U. arrecta dominance and the functional diversity indices in each sampling period, we found 21 occasions (in a total universe of 40, because: 5 indexes × 8 sampling periods) when the indices differed in respect to the categories of dominance (Figs. S8–S12 in Appendix). On 17 occasions, reduced values were from the category ‘dominant’; increased values from the category ‘absent’ occurred on 10 occasions. Regarding the sensibility of the indexes, Fdis, Fdiv and RaoQ entropy had differences among dominance categories in five occasions, Feve in four and Fric in one.
The dominance of U. arrecta also effected the distances to centroid, both taxonomic (F1,3 = 19.7941, P < 0.0001) and functional (F1,3 = 7.9309, P = 0.0001). After pooling the data, and controlling by the sampling period, sites without U. arrecta had higher variances among themselves than the sites where U. arrecta was dominant (Fig. S13 in Appendix). When testing for the effects of U. arrecta abundance on the community’s variance for each sampling period separately, there were elevated distances to the centroid associated with sites without U. arrecta in all samplings that took place in winter, and none in summer, for taxonomic data (Fig. 9). The CWM matrix data also showed differences among categories of dominance but only in Sep/16 and Sep/17, between ‘dominant’ and ‘mangrove’ categories, and ‘dominant’ and the other categories, respectively (Fig. S14 in Appendix).
Taxonomic dispersion among sites according to the Urochloa arrecta (Hack. ex T. Durand & Schinz) Morrone & Zuloaga dominance classes (see methods). The asterisks represent P < 0.05 in unequal variances according to PERMUTEST, centroids are highlighted using larger-sized symbols. April/16–April/19 indicate April 2016–April 2019; and Sep/16–Sep/19 indicate September 2016–September 2019
The indicator species associated with each dominance category are shown in Table 2. In general, the species associated with sites where U. arrecta was absent were emergent; those associated with sites where U. arrecta was dominant were free floating. The most diverse class regarding life form was the ‘not dominant’, where emergent, submersed floating, emergent floating and epiphyte species were associated.
Discussion
Here, we conducted an investigation of spatial and temporal variation of macrophyte taxonomic and functional diversity in a subtropical tidal river, and revealed interesting patterns. Although seasonality in this subtropical environment is well marked, macrophytes respond in a very complex way to seasonal variation. Neither community composition nor compositional variation differed between seasons when compared to the whole river. Instead, dissimilarity between subsequent seasons seems to be decreasing over the four-years of standardized monitoring, both for taxonomic and functional facet of the macrophyte metacommunity. Even so, one seasonal pattern was observed: the spatial differences in community composition were high after winter. Yet, some spatial patterns were consistent over time: community structure had a clear river-continuum autocorrelation, and consistently differed according to the invasive U. arrecta dominance in this tidal coastal river.
The seasonal changes in rainfall also summarize differences regarding total nutrient input (Mizerkowski et al. 2012), particularly given the higher human impacts in summer holidays associated to enhanced population density. As a subtropical environment, temperature obviously has a seasonal pattern, but it is unlikely that relatively lower temperatures in winter affect plant growth, given the average values at coldest months is c. 18 °C (Vanhoni and Mendonça 2008). Even though rainfall data had a seasonal pattern, accumulated rainfall data from 15 days before the field survey had no relationship with biodiversity.
The occurrence of seasonal variation in macrophyte community was expected based on the above-mentioned seasonality in rainfall. In summer, the resulting community may probably be shaped by a higher dispersion of individuals and changes in establishment, due the higher rainfall and temperature, and the increased amounts of nutrients in the water. The higher dispersion and nutrient input in the water would be especially important for floating species (Trindade et al. 2018; Hussner et al. 2021). The patterns in traits highlighted by the use of TBI approach on CWM matrix data corroborate our hypotheses. The increase in leaf width in summer might be a result of nutrient stimulus to growth, as larger leaves allow the capture of more sunlight and, therefore, increased biomass production (Chambers et al. 2008); or a response to enhanced water flow (Franklin et al. 2008). The increase in foliar area usually seen in summer is consistent with this idea, although the pattern of increases and decreases was not as consistent as that for leaf width. The level of herbivory on leaves did not change with time, suggesting that other interactions and factors are probably more important in the temporal dynamics of these communities, such as physical and chemical conditions of water (e.g. Lindholm et al. 2020) or underwater herbivory (e.g., Bakker et al. 2016). The increased stem width associated, in general, with winter is a difficult trait to associate to possible seasonal changes, because it is a species-specific response (Puijalon and Bornette 2004) and can be associated to other factors, such as water flow (Bociąg et al. 2009) and nutrients (Lamberti-Raverot and Puijalon 2012), in opposite directions; but it may also mean a trade-off of less investment in leaves and more investment in storage after the relatively harsher conditions of winter (see also Cornelissen and Makoto 2014). Even though our empirical data allow us to speculate, only detailed environmental data and experiments would determine exactly which factors are influencing seasonal differentiation in traits (Jiang et al. 2008; Stefanidis and Papastergiadou 2019).
The variability of functional indexes of alpha diversity reinforces the results of other studies using more than one approach to evaluate the biodiversity (Heino et al. 2007; Villéger et al. 2012; Trigal et al. 2014). Beyond choosing the right index—according to its formulation, purpose and sensitivity—, we argue that uncovering which specific traits vary or remain the same is a very useful link between environmental conditions, species response and ecosystem functioning. In our experience, the TBI approach applied to the CWM matrix was promising and showed interesting patterns discussed above and below.
The seasonal effects described above are also corroborated by considering together the trend of increase in number of shared species and reduction in the variance (i.e., distances to centroid) among sites in summer, and the increase in the gain component (C) of TBI in transitions from winter to summer. This high similarity associated to summer is expected considering the homogenising effects when the ecosystem has disturbances (see Thomaz et al. 2007), which in our case are those associated to increased rainfall.
As stated before, even with the increase in abundance and number of species in summer, it was still possible to observe a spatial structuring of the river into different sectors. Taking into consideration the interplay between spatial and temporal dynamics, we suggest that, after winter, the local factors spatially structuring the river probably increase in importance. The rationale for that is the reduction in homogenisation and nutrient supply in the water, promoting differentiation between the communities as the environmental conditions become more dissimilar (Thomaz et al 2007). An interesting issue to be studied in the future is how long does it take for these seasonal variations to occur. One might hypothesize that the changes from winter to summer are faster than changes from summer to winter because the populations may respond quicker to increased temperature, nutrients and light (see also Lindholm et al. 2020).
It was interesting to describe the complex temporal changes: whereas the betadisper approach did not show temporal changes, the TBI indicated that the sites, individually, had a reduction in diversity during the studied period. This temporal reduction, despite the absence of any clear evidence of changes in general trend of rainfall during the studied period (Fig. S15 in Appendix), might indicate a trend toward biotic homogenisation. Biotic homogenisation was firstly introduced as a continuous temporal trend of decreasing biodiversity due to many ‘loser’ and few ‘winner’ species (McKinney and Lockwood 1999). As research evidence accumulated, different facets of biotic homogenisation phenomenon were reported (Olden et al. 2018; Padial et al. 2020). Here, we present another interesting facet: the temporal decrease in seasonal variation, i.e., the seasonal homogenisation. We suggest that this is a concerning facet of biotic homogenisation, given that other organisms that depend on seasonal variation of macrophytes may also be affected, decreasing the biodiversity at long term. Moreover, we also found that spatial differences among the classes of U. arrecta dominance (discussed in details below) also depend on seasonality. If macrophyte community organization and biodiversity became more similar between seasons, so a higher constancy in the effects of the invasive U. arrecta may also be expected.
Effects of the invasive tanner grass scrutinized
In addition to the well-known impact of the gradient in environmental conditions along the river on the structure of the communities, our results clearly demonstrated that distribution of species and biodiversity indexes respond to the dominance of an invasive species. Indeed, the effect of U. arrecta on aquatic macrophytes in Guaraguaçu River showed clear spatial and temporal components. The spatial effects include the, until now, absence of this invasive species in the ‘Caixetal’ (upper river) and Mangrove (down river) ecosystems. In the particular case of the ‘Caixetal’, we do not know whether physiological tolerances, the lack of opportunities (dispersion of enough propagules upward the river) and/or the more preserved terrestrial vegetation prevent its establishment in this area (Evangelista et al. 2017; Teixeira et al. 2016; but see Pott et al. 2011 reporting U. arrecta invasion in presence of Tabebuia insignis (Miq.) Sandw.). This component of spatial structure makes it more complicated to disentangle which are the main factors promoting the higher dissimilarities among the sites without U. arrecta: if it is its absence or other factors (e.g., physical and chemical conditions) that we did not measure. Even so, a previous study comparing neighbouring macrophyte beds have also demonstrated that U. arrecta decreased biodiversity and promoted compositional changes at different spatial scales (Sato et al. 2021). The analyses on our monitoring data reinforce this conclusion and add that U. arrecta effects extend to the entire river length. This is of particular concern given that a previous study has demonstrated that this invasive species can show rapid adaptive responses to cope with increased salinity (Bora et al. 2020), so the overwhelming effect of the invasive species can extend toward the mangrove, central areas for conservation in the Lagamar region (Araújo et al. 2021). After nuisance growth, management and control efforts are complex and in most cases, not effective (Hussner et al. 2017).
It is interesting to note that the temporal dimension of the effects of U. arrecta on the system is the variation of the distances to centroid in a direction opposite to that seen in the sites where U. arrecta is absent. In winter, the sites categorized as ‘dominant’ are more similar to each other than in summer. This opposite direction of increase and decrease between sites where U. arrecta is dominant and where it is absent, probably explains why the significant differences in distance to centroids among these categories occurred in winter and not summer. This indicates that more than affecting spatial community structuring (Sato et al. 2021) the invasive tanner grass can also change the temporal dynamics of macrophyte communities. We hypothesized two main factors, acting in conjunction, for the enhanced similarity among sites where U. arrecta dominates in winter. The first is the supposedly more frequent removal of beds of U. arrecta because of higher water flows due to rainfall in summer, releasing space for the establishment of other plants. In this scenario, the main limiting resource macrophytes are competing for would be space. The second is the known and widely reported high tolerance and rapid evolution of U. arrecta to a wide range of environmental conditions (Bora et al. 2020). This high tolerance, together with its high growth rate (Amorim et al. 2015; Michelan et al. 2010; Thomaz et al. 2009, 2012), make this species one of the most potentially invasive plant species known (Michelan et al. 2010). Indeed, traits related to nutrient absorption in invasive species, for instance, are commonly associated to invasiveness (Hussner et al. 2021). In winter, the total nutrient load in the river is lower due to the reduced water flow (Mizerkowski et al. 2012). Under this likely limiting condition in winter, the invasive U. arrecta could have an advantage over the other aquatic macrophytes, in addition to shaping the beds’ local environmental features that could reduce the chances of establishment of other species. One example of seasonal change is the progression of its biomass towards the pelagic zone, resulting from its growth above its own biomass, leaving no space in the littoral zone for other emergent plants (Bao et al. 2015). The presence of free-floating species on beds where U. arrecta is dominant corroborates this rationale. Yet, the decomposition of U. arrecta biomass and the diminished circulation of water between its stems, could favour an accumulation of nutrients in the adjacent water, allowing the free-floating plants to prosper after ending up trapped by the extensive U. arrecta macrophyte beds (e.g., Dainez-Filho et al. 2019). Furthermore, the dominance of the established U. arrecta can hamper the recolonization of other species (Michelan et al. 2018), promoting a positive feedback.
Our results corroborate many previous studies that reported a negative impact of U. arrecta on the diversity of plant communities (Thomaz and Cunha 2010; Fernandes et al. 2013; Bao et al. 2015; Sato et al. 2021), and show that even the beds dominated by U. arrecta are subjected to some seasonal variation. Even so, it is important to mention that the main homogenising effect of U. arrecta was probably observed in the first years after its establishment in the river (see e.g. Vitule et al. 2007). Since the first sampling period, the extensive U. arrecta beds were already established over all river (A.A.P. personal observation; see also Araújo et al. 2021). It is important to highlight though that expansions towards other river stretches such as the mangrove is possible, since this species demonstrates rapid evolutionary responses to salinity (Bora et al. 2020). We here reinforced the threat to biodiversity promoted by U. arrecta and the seasonal component of this threat. The continuing monitoring is central to reveal how metacommunities will evolve in Guaraguaçu River ecosystem (see also Hussner 2014).
Conclusions
Results summarized in this study are the first from a long-term monitoring program in Guaraguaçu River. A temporal pattern in biodiversity was found, in which more species are shared among the sampled sites in summer than in winter. As a consequence, the traits of the communities also tended to vary more in winter than summer. We suggest the rainfall accumulated over the seasons is the main driver of changes in environmental conditions, which will shape locally the communities over time. We hypothesize that in winter, the communities are under more limiting conditions, and local specific characteristics become more important, favouring higher differentiation among the communities. This alternation or fluctuation in aquatic environments is a key factor driving the food web in these environments (e.g. Contente et al. 2011b), and changes in the patterns can impact the whole ecosystem (Maltchik et al. 2007). Thus, by understanding the temporal dynamics of the Guaraguaçu River communities and the dominance of U. arrecta, impacts of future scenarios, such as severe events (e.g., droughts) or changes in the distribution of rainfall during the year, can be estimated. In particular, it will be important to assess how individuals will respond to the interaction of different environmental cues, such as a result of climate change (e.g., Fukushima et al. 2021). Finally, we showed the negative effects of the invasive U. arrecta are also influenced by the season, and its effects are more evident in winter, although observed in all periods. Studying the consequences for the invasive U. arrecta is necessary to explain community and ecosystem modification related to other biological groups and process important to humans and future generations.
Data availability statement
The datasets generated during and/or analyzed during the current study are available at https://lasbufprbio.wixsite.com/home or from the corresponding author on reasonable request.
References
Alahuhta J, Rosbakh S, Chepinoga V, Heino J (2020) Environmental determinants of lake macrophyte communities in Baikal Siberia. Aquat Sci 82:39. https://doi.org/10.1007/s00027-020-0710-8
Amorim SR, Umetsu CA, Camargo AFM (2015) Effects of a non-native species of Poaceae on aquatic macrophyte community composition: a comparison with a native species. J Aquat Plant Manag 53:191–196
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
APG III (2009) Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121. https://doi.org/10.1111/j.1095-8339.2009.00996.x
Araújo ES, Vitule JRS, Padial AA (2021) A checklist of aquatic macrophytes of the Guaraguaçu River basin reveals a target for conservation in the Atlantic rainforest. Acta scientiarum. Biol Sci 43(1):e50542. https://doi.org/10.4025/actascibiolsci.v43i1.50542
Bakker ES, Pagès JF, Arthur R, Alcoverro T (2016) Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39:162–179. https://doi.org/10.1111/ecog.01651
Bao F, Assis MA, Arruda R, Pott A (2015) Effects of Urochloa humidicola on plant diversity in native grasslands in a neotropical wetland. Wetlands 35:841–850. https://doi.org/10.1007/s13157-015-0673-z
Bociąg K, Galka A, Lazarewicz T, Szmeja J (2009) Mechanical strength of stems in aquatic macrophytes. Acta Soc Bot Pol 78:181–187
Bolpagni R, Lastrucci L, Brundu G, Hussner A (2020) Editorial: multiple roles of alien plants in aquatic ecosystems: from processes to modelling. Front Plant Sci 11:1299. https://doi.org/10.3389/fpls.2020.01299
Bora LS, Thomaz SM, Padial AA (2020) Evidence of rapid evolution of an invasive poaceae in response to salinity. Aquat Sci 82:1–16. https://doi.org/10.1007/s00027-020-00750-y
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73(1):1–14. https://doi.org/10.1007/s00027-010-0162-7
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087. https://doi.org/10.1111/j.1365-2664.2011.02048.x
Cavalli G, Baattrup-Pedersen A, Riis T (2014) The role of species functional traits for distributional patterns in lowland stream vegetation. Freshw Sci 33:1074–1085
Ceschin F, Bini LM, Padial AA (2017) Correlates of fish and aquatic macrophyte beta diversity in the Upper Paraná River floodplain. Hydrobiologia 805(1):377–389. https://doi.org/10.1007/s10750-017-3325-x
Chambers PA, Vis C, Brua RB, Guy M, Culp JM, Benoy GA (2008) Eutrophication of agricultural streams: defining nutrient concentrations to protect ecological condition. Wat Sci Technol 58(11):2203–2210. https://doi.org/10.2166/wst.2008.815
Contente RF, Stefanoni MF, Spach HL (2011a) Fish assemblage structure in an estuary of the Atlantic Forest biodiversity hotspot (southern Brazil). Ichthyol Res 58(1):38–50. https://doi.org/10.1007/s10228-010-0192-0
Contente RF, Stefanoni MF, Spach HL (2011b) Feeding ecology of the brazilian silverside Atherinella brasiliensis (Atherinopsidae) in a sub-tropical estuarine ecosystem. J Mar Biol Assoc UK 91(6):1197–1205. https://doi.org/10.1017/S0025315410001116
Cornelissen JH, Makoto K (2014) Winter climate change, plant traits and nutrient and carbon cycling in cold biomes. Ecol Res 29:517–527. https://doi.org/10.1007/s11284-013-1106-1
Dainez-Filho MS, Michelan TS, Louback-Franco N, Souza DC, Cafofo EG, Thomaz SM (2019) Role of sediment structuring by detritus on colonization and interspecific competition of one native and one invasive submerged macrophyte. Hydrobiologia 834(1):63–74. https://doi.org/10.1007/s10750-019-3909-8
Dufrene M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67(3):345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Elo M, Alahuhta J, Kanninen A, Meissner KK, Seppälä K, Mönkkönen M (2018) Environmental characteristics and anthropogenic impact jointly modify aquatic macrophyte species diversity. Front Plant Sci 9:1001. https://doi.org/10.3389/fpls.2018.01001
Evangelista HB, Michelan TS, Gomes LC, Thomaz SM (2017) Shade provided by riparian plants and biotic resistance by macrophytes reduce the establishment of an invasive Poaceae. J Appl Ecol 54:648–656
Faria L, Frehse FA, Occhi TVT, Carvalho BM, Pupo DV, Disaró ST, Vitule JRS (2021) Occurrence of non-native species in a subtropical coastal River, in Southern Brazil. Acta Limnol Bras 33:e101. https://doi.org/10.1590/S2179-975X2320
Fernandes LFG, Teixeira MC, Thomaz SM (2013) Diversity and biomass of native macrophytes are negatively related to dominance of an invasive Poaceae in Brazilian sub-tropical streams. Acta Limnol Bras 25:202–209. https://doi.org/10.1590/s2179-975x2013000200011
Flynn DFB, Mirotchnick N, Jain M, Palmer MI, Naeem S (2011) Functional and phylogenetic diversity as predictors of biodiversity-ecosystem function relationships. Ecology 92:1573–1581. https://doi.org/10.1890/10-1245.1
Franklin P, Dunbar M, Whitehead P (2008) Flow controls on lowland river macrophytes: a review. Sci Total Environ 400:369–378. https://doi.org/10.1016/j.scitotenv.2008.06.018
Fritz C, Schneider T, Geist J (2017) Seasonal variation in spectral response of submerged aquatic macrophytes: a case study at Lake Starnberg (Germany). Water 9(521):1–21. https://doi.org/10.3390/w9070527
Fu H, Zhong J, Yuan G, Ni L, Xie P, Cao T (2014) Functional traits composition predict macrophytes community productivity along a water depth gradient in a freshwater lake. Ecol Evol 4(9):1516–1523. https://doi.org/10.1002/ece3.1022
Fukushima K, Narukawa H, Palfalvi G, Hasebe M (2021) A discordance of seasonally covarying cues uncovers misregulated phenotypes in the heterophyllous pitcher plant Cephalotus follicularis. Proc R Soc B 288:20202568. https://doi.org/10.1098/rspb.2020.2568
Haller WT, Sutton DL, Barlowe WC (1974) Effects of salinity on growth of several aquatic macrophytes. Ecology 55(4):891–894. https://doi.org/10.2307/1934427
Havel JE, Kovalenko KE, Thomaz SM, Amalfitano S, Kats LB (2015) Aquatic invasive species: challenges for the future. Hydrobiologia 750(1):147–170. https://doi.org/10.1007/s10750-014-2166-0
Heino J, Mykrä H, Kotanen J, Muotka T (2007) Ecological filters and variability in stream macroinvertebrate communities: do taxonomic and functional structure follow the same path? Ecography 30:217–230. https://doi.org/10.1111/j.2007.0906-7590.04894.x
Hussner A (2014) Long-term macrophyte mapping documents a continuously shift from native to non-native aquatic plant dominance in the thermally abnormal River Erft (North Rhine-Westphalia, Germany). Limnologica 48:39–45. https://doi.org/10.1016/j.limno.2014.05.003
Hussner A, Stiers I, Verhofstad MJJM, Bakker ES, Grutters BMC, Haury J, van Valkenburg JLCH, Brundu G, Newman J, Clayton JS, Anderson LWJ, Hofstra D (2017) Management and control methods of invasive alien freshwater aquatic plants: a review. Aquat Bot 136:112–137. https://doi.org/10.1016/j.aquabot.2016.08.002
Hussner A, Heidbüchel P, Coetzee J, Gross EM (2021) From introduction to nuisance growth: a review of traits of alien aquatic plants which contribute to their invasiveness. Hydrobiologia 848:2119–2151. https://doi.org/10.1007/s10750-020-04463-z
IPARDES, Instituto Paranaense de Desenvolvimento Econômico e Social (2013) Indicadores de desenvolvimento sustentável por bacias hidrográficas do Estado do Paraná. IPARDES, Curitiba. http://www.ipardes.pr.gov.br/Pagina/Indicadores-de-Desenvolvimento-Sustentavel. Accessed 3 Apr 2021
Jiang J, Zhou C, An S, Yang H, Guan B, Cai Y (2008) Sediment type, population density and their combined effect greatly charge the short-time growth of two common submerged macrophytes. Ecol Eng 34:79–90. https://doi.org/10.1016/j.ecoleng.2008.07.003
Lacoul P, Freedman B (2006) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14(2):89–136. https://doi.org/10.1139/A06-001
Laliberté E, Legendre P (2010) A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. https://doi.org/10.1890/08-2244.1
Lamberti-Raverot B, Puijalon S (2012) Nutrient enrichment affects the mechanical resistance of aquatic plants. J Exp Bot 63(17):6115–6123. https://doi.org/10.1093/jxb/ers268
Lana PC, Marone EL, Lopes RM, Machado EC (2001) The subtropical estuarine complex of Paranaguá Bay, Brazil. In: Seeliger U, Lacerda LD, Kjerfve BJ (eds) Coastal marine ecosystems of Latin America. Springer, Berlin, pp 131–145
Legendre P (2019) A temporal beta-diversity index to identify sites that have changed in exceptional ways in space–time surveys. Ecol Evol 9(6):3500–3514. https://doi.org/10.1002/ece3.4984
Lindholm M, Alahuhta J, Heino J, Toivonen H (2020) Temporal beta diversity of lake plants is determined by concomitant changes in environmental factors across decades. J Ecol. https://doi.org/10.1111/1365-2745.13508
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world's worst invasive alien species: a selection from the global invasive species database. Auckland: Invasive Species Specialist Group. https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf. Assessed 4 Aug 2021.
Magnusson WE, Lima AP, Luizão R, Luizão F, Costa FRC, Castilho CV, Kinupp VF (2005) RAPELD: a modification of the gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotrop 5(2):1–6. https://doi.org/10.1590/S1676-06032005000300002
Maltchik L, Rolon AS, Schott P (2007) Effects of hydrological variation on the aquatic plant community in a floodplain palustrine wetland of southern Brazil. Limnology 8:23–28. https://doi.org/10.1007/s10201-006-0192-y
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14(11):450–453. https://doi.org/10.1016/S0169-5347(99)01679-1
Michelan TS, Thomaz SM, Mormul RP, Carvalho P (2010) Effects of an exotic invasive macrophyte (tropical signalgrass) on native plant community composition, species richness and functional diversity. Freshw Biol 55:1315–1326. https://doi.org/10.1111/j.1365-2427.2009.02355.x
Michelan TS, Thomaz SM, Bini LM (2013) Native macrophyte density and richness affect the invasiveness of a tropical poaceae species. PLoS ONE 8(3):1–8. https://doi.org/10.1371/journal.pone.0060004
Michelan TS, Thomaz SM, Bando FM, Bini LM (2018) Competitive effects hinder the recolonization of native species in environments densely occupied by one invasive exotic species. Front Plant Sci 9:1261. https://doi.org/10.3389/fpls.2018.01261
Mizerkowski BD, Hesse KJ, Ladwig N, da Costa ME, Rosa R, Araujo T, Koch D (2012) Sources, loads and dispersion of dissolved inorganic nutrients in Paranaguá Bay. Ocean Dyn 62:1409–1424. https://doi.org/10.1007/s10236-012-0569-x
Moura-Júnior EG, Lima LF, Silva SSL, Paiva RMS, Ferreira FA, Zickel CS, Pott A (2015) Aquatic macrophytes of Northeastern Brazil: checklist, richness, distribution and life forms. Check List 9(2):298–312. https://doi.org/10.15560/9.2.298
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Olden JD, Comte L, Giam X (2018) The homogocene: a research prospectus for the study of biotic homogenisation. NeoBiota 37:23. https://doi.org/10.3897/neobiota.37.22552
Padial AA, Vitule JRS, Olden JD (2020) Preface: aquatic homogenocene—understanding the era of biological re-shuffling in aquatic ecosystems. Hydrobiologia 847(18):3705–3709. https://doi.org/10.1007/s10750-020-04413-9
Pavão AC, Santos ACA, Bottino F, Benassi RF, Calijuri MC (2017) Richness and distribution of aquatic macrophytes in a subtropical reservoir in São Paulo, Brazil. Acta Limnol Bras 29(10):1–11. https://doi.org/10.1590/S2179-975X7016
Pedralli G (1990) Macrófitos aquáticos: técnicas e métodos de estudos. Estudos De Biologia 26:5–24
Petchey OL, Gaston KJ (2006) Functional diversity: back to basics and looking forward. Ecol Lett 9:741–758. https://doi.org/10.1111/j.1461-0248.2006.00924.x
Pinto RC, Passos E, Caneparo SC (2012) Classificação dos movimentos de massa ocorridos em março de 2011 na Serra da Prata, estado do Paraná. Geoingá 4(1):3–27. https://doi.org/10.4025/geoinga.v4i1.49152
Pott VJ, Pott A (2000) Plantas aquáticas do Pantanal. Brasília: EMBRAPA
Pott VJ, Pott A, Lima LCP, Moreira SN, Oliveira AKM (2011) Aquatic macrophyte diversity of the Pantanal wetland and upper basin. Braz J Biol 71(1):255–263. https://doi.org/10.1590/S1519-69842011000200004
Pozo R, Fernández-Aláez C, Fernández-Aláez M (2011) The relative importance of natural and anthropogenic effects on community composition of aquatic macrophytes in Mediterranean ponds. Mar Freshw Res 62(2):101–109. https://doi.org/10.1071/MF10125
Puijalon S, Bornette G (2004) Morphological variation of two taxonomically distant plant species along a natural flow velocity gradient. New Phytol 163:651–660. https://doi.org/10.1111/j.1469-8137.2004.01135.x
QGIS Geographic Information System (2021) QGIS Association. http://www.qgis.org. Accessed 31 Mar 2022
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 31 Mar 2022
Rolon AS, Homem HF, Maltchik, (2010) Aquatic macrophytes in natural and managed wetlands of Rio Grande do Sul State. Southern Brazil. Acta Limnol Bras 22(2):133–146. https://doi.org/10.4322/actalb.02202003
Sato RY, Costa APL, Padial AA (2021) The invasive tropical tanner grass decreases diversity of the native aquatic macrophyte community at two scales in a subtropical tidal river. Acta Bot Bras 35(1):140–150. https://doi.org/10.1590/0102-33062020abb0360
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28(1):58–66. https://doi.org/10.1016/j.tree.2012.07.013ff
Song Y, Wang P, Li G, Zhou D (2014) Relationships between functional diversity and ecosystem functioning: a review. Acta Ecol Sinica 34(2):85–91. https://doi.org/10.1016/j.chnaes.2014.01.001
Stefanidis K, Papastergiadou E (2019) Linkages between macrophyte functional traits and water quality: Insights from a study in freshwater lakes of Greece. Water 11(5):1047. https://doi.org/10.3390/w11051047
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21(11):645–651. https://doi.org/10.1016/j.tree.2006.07.007
Teixeira MC, Bini LM, Thomaz SM (2016) Biotic resistance buffers the effects of nutrient enrichment on the success of a highly invasive aquatic plant. Freshw Biol 62(1):65–71. https://doi.org/10.1111/fwb.12849
Thomaz SM, Cunha ER (2010) The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol Bras 22(2):218–236. https://doi.org/10.4322/actalb.02202011
Thomaz SM, Bini LM, Bozelli RL (2007) Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579(1):1–13. https://doi.org/10.1007/s10750-006-0285-y
Thomaz SM, Carvalho P, Mormul RP, Ferreira FA, Silveira MJ, Michelan TS (2009) Temporal trends and effects of diversity on occurrence of exotic macrophytes in a large reservoir. Acta Oecol 35:614–620. https://doi.org/10.1016/j.actao.2009.05.008
Thomaz SM, Silveira MJ, Michelan TS (2012) The colonization success of an exotic Poaceae is related to native macrophyte richness, wind disturbance and riparian vegetation. Aquat Sci 74(4):809–815. https://doi.org/10.1007/s00027-012-0264-5
Thomaz SM, Kovalenko KE, Havel JE, Kats LB (2015) Aquatic invasive species: general trends in the literature and introduction to the special issue. Hydrobiologia 746(1):1–12. https://doi.org/10.1007/s10750-014-2150-8
Trigal C, Fernandez-Alaez C, Fernandez-Alaez M (2014) Congruence between functional and taxonomic patterns of benthic and planktonic assemblages in flatland ponds. Aquat Sci 76:61–72. https://doi.org/10.1007/s00027-013-0312-9
Trindade CRT, Landeiro VL, Schneck F (2018) Macrophyte functional groups elucidate the relative role of environmental and spatial factors on species richness and assemblage structure. Hydrobiologia 823:217–230. https://doi.org/10.1007/s10750-018-3709-6
Vanhoni F, Mendonça F (2008) O clima do litoral do estado do Paraná. Rev Bras Clim. https://doi.org/10.5380/abclima.v3i0.25423
Vecchia AD, Villa P, Bolpagni R (2020) Functional traits in macrophyte studies: current trends and future research agenda. Aquat Bot. https://doi.org/10.1016/j.aquabot.2020.103290
Villéger S, Miranda JR, Hernandez DF, Mouillot D (2012) Low functional β-diversity despite high taxonomic β-diversity among tropical estuarine fish communities. PLoS ONE 7(7):e40679. https://doi.org/10.1371/journal.pone.0040679
Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007) Let the concept of trait be functional! Oikos 116:882–892. https://doi.org/10.1111/j.0030-1299.2007.15559.x
Vitule JRS, Umbria SC, Aranha JMR (2006) Introduction of the African catfish Clarias gariepinus (Burchell, 1822) into Southern Brazil. Biol Invas 8:677–681. https://doi.org/10.1007/s10530-005-2535-8
Vitule JRS (2007) Distribuição, abundância e estrutura populacional de peixes introduzidos no Rio Guaraguaçu, Paranaguá, Paraná, Brasil. Thesis. Universidade federal do Paraná
Acknowledgements
The authors thank CAPES for the scholarships for E.F.G, A.P.L.C. and E.S.A (financial code 001); and CNPq continuous financial supports for J.R.S.V. and A.A.P that contributed to the project (Project numbers: 310850/2012-6; 303776/2015-3; 307984/2015-0; 402828/2016-0; 301867/2018-6). All authors presented substantial contribution to the concept, design and preparation of the manuscript. Authors also acknowledge students of ‘Laboratório de Análise e Síntese em Biodiversidade’ <https://lasbufprbio.wixsite.com/home> and ‘Laboratório de Ecologia e Conservação’ <https://vitulelab.wixsite.com/lec-ufpr> for help in field samplings. Data generated in this manuscript is a result of long-term monitoring designed by A.A.P and J.R.S.V. that started in 2016. Data is freely available for the use by academic community at <https://lasbufprbio.wixsite.com/home>, which demonstrate the importance of national and international agencies in supporting initiatives of biodiversity monitoring.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galvanese, E.F., Costa, A.P.L., Araújo, E.S. et al. Community stability and seasonal biotic homogenisation emphasize the effect of the invasive tropical tanner grass on macrophytes from a highly dynamic neotropical tidal river. Aquat Sci 84, 30 (2022). https://doi.org/10.1007/s00027-022-00858-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-022-00858-3