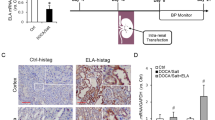
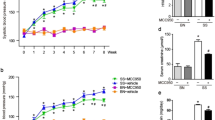
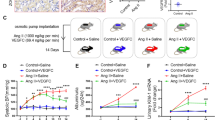
Abstract
Salt-sensitivity hypertension (SSHTN) is an independent predictor for cardiovascular mortality. VEGFC has been reported to be a protective role in SSHTN and hypertensive kidney injury. However, the underlying mechanisms remain largely unclear. The current study aimed to explore the protective effects and mechanisms of VEGFC against SSHTN and hypertensive nephropathy. Here, we reported that VEGFC attenuated high blood pressure as well as protected against renal inflammation and fibrosis in SSHTN mice. Moreover, VEGFC suppressed the activation of renal NLRP3 inflammasome in SSHTN mice. In vitro, we found VEGFC inhibited NLRP3 inflammasome activation, meanwhile, upregulated autophagy in high-salt-induced macrophages, while these effects were reversed by an autophagy inhibitor 3MA. Furthermore, in vivo, 3MA pretreatment weakened the protective effects of VEGFC on SSHTN and hypertensive nephropathy. Mechanistically, VEGF receptor 3 (VEGFR3) kinase domain activated AMPK by promoting the phosphorylation at Thr183 via binding to AMPK, thus enhancing autophagy activity in the context of high-salt-induced macrophages. These findings indicated that VEGFC inhibited NLRP3 inflammasome activation by promoting VEGFR3-AMPK-dependent autophagy pathway in high-salt-induced macrophages, which provided a mechanistic basis for the therapeutic target in SSHTN and hypertensive kidney injury.
Graphical abstract









Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Availability of data and materials
Supplementary data to this article can be found online.
References
Brouwers S, Sudano I, Kokubo Y, Sulaica EM (2021) Arterial hypertension. Lancet (London, England) 398(10296):249–261. https://doi.org/10.1016/s0140-6736(21)00221-x
Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS (1986) Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 8(6 Pt 2):Ii127–Ii134. https://doi.org/10.1161/01.hyp.8.6_pt_2.ii127
Weinberger MH (1996) Salt sensitivity of blood pressure in humans. Hypertension 27(3 Pt 2):481–490. https://doi.org/10.1161/01.hyp.27.3.481
Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G (1997) Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350(9093):1734–1737. https://doi.org/10.1016/s0140-6736(97)05189-1
Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M (2001) Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37(2 Pt 2):429–432. https://doi.org/10.1161/01.hyp.37.2.429
McMaster WG, Kirabo A, Madhur MS, Harrison DG (2015) Inflammation, immunity, and hypertensive end-organ damage. Circul Res 116(6):1022–1033. https://doi.org/10.1161/circresaha.116.303697
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN (2017) Salt-responsive gut commensal modulates T(H)17 axis and disease. Nature 551(7682):585–589. https://doi.org/10.1038/nature24628
Mattson SD (2019) Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 15(5):290–300. https://doi.org/10.1038/s41581-019-0121-z
Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, Whelton PK (2018) Hypertension. Nat Rev Dis Primers 4:18014. https://doi.org/10.1038/nrdp.2018.14
Mutchler SM, Kirabo A, Kleyman TR (2021) Epithelial sodium channel and salt-sensitive hypertension. Hypertension 77(3):759–767. https://doi.org/10.1161/hypertensionaha.120.14481
Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176(6):248–1264. https://doi.org/10.1016/j.cell.2019.01.021
Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15(5):545–552. https://doi.org/10.1038/nm.1960
Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, Lopez AH, Hudson KR, Johnson ER, Muthuchamy M, Mitchell BM, Rutkowski JM (2018) Enhancing renal lymphatic expansion prevents hypertension in mice. Circul Res 122(8):1094–1101. https://doi.org/10.1161/circresaha.118.312765
Glinton KE, Ma W, Lantz C, Grigoryeva LS, DeBerge M, Liu X, Febbraio M, Kahn M, Oliver G, Thorp EB (2022) Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J Clin Investig. https://doi.org/10.1172/jci140685
Justin Rucker A, Crowley SD (2017) The role of macrophages in hypertension and its complications. Pflugers Archiv 469(3–4):419–430. https://doi.org/10.1007/s00424-017-1950-x
Barbaro NR, Fontana V, Modolo R, De Faria AP, Sabbatini AR, Fonseca FH, Anhê GH, Moreno H (2015) Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Pressure 24(1):7–13. https://doi.org/10.3109/08037051.2014.940710
Dörffel Y, Lätsch C, Stuhlmüller B, Schreiber S, Scholze S, Burmester GR, Scholze J (1999) Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 34(1):113–117. https://doi.org/10.1161/01.hyp.34.1.113
Krishnan SM, Dowling JK, Ling YH, Diep H, Chan CT, Ferens D, Kett MM, Pinar A, Samuel CS, Vinh A, Arumugam TV, Hewitson TD, Kemp-Harper BK, Robertson AA, Cooper MA, Latz E, Mansell A, Sobey CG, Drummond GR (2016) Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 173(4):752–765. https://doi.org/10.1111/bph.13230
Rodriguez-Iturbe B, Pons H, Johnson RJ (2017) Role of the immune system in hypertension. Physiol Rev 97(3):1127–1164. https://doi.org/10.1152/physrev.00031.2016
Pitzer A, Elijovich F, Laffer CL, Ertuglu LA, Sahinoz M, Saleem M, Krishnan J, Dola T, Aden LA, Sheng Q, Raddatz MA, Wanjalla C, Pakala S, Davies SS, Patrick DM, Kon V, Ikizler TA, Kleyman T, Kirabo A (2022) ENaC-dependent inflammasome activation contributes to salt-sensitive hypertension. Circul Res 131(4):328–344. https://doi.org/10.1161/circresaha.122.320818
Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AMK (2015) mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep 12(1):102–115. https://doi.org/10.1016/j.celrep.2015.05.046
Tian X, Yu C, Shi L, Li D, Chen X, Xia D, Zhou J, Xu W, Ma C, Gu L, An Y (2018) MicroRNA-199a-5p aggravates primary hypertension by damaging vascular endothelial cells through inhibition of autophagy and promotion of apoptosis. Exp Therap Med 16(2):595–602. https://doi.org/10.3892/etm.2018.6252
Stanton MJ, Dutta S, Zhang H, Polavaram NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH, Datta K (2013) Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance. Cancer Res 73(1):160–171. https://doi.org/10.1158/0008-5472.Can-11-3635
Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ, Rodríguez-Iturbe B (2001) Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281(1):F38–F47. https://doi.org/10.1152/ajprenal.2001.281.1.F38
Wu C, Chen H, Zhuang R, Zhang H, Wang Y, Hu X, Xu Y, Li J, Li Y, Wang X, Xu H, Ni W, Zhou K (2021) Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci 17(4):1138–1152. https://doi.org/10.7150/ijbs.57825
Liu X, Cao H, Li J, Wang B, Zhang P, Dong Zhang X, Liu Z, Yuan H, Zhan Z (2017) Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination. Cell Death Differ 24(4):683–693. https://doi.org/10.1038/cdd.2017.1
Beaini S, Saliba Y, Hajal J, Smayra V, Bakhos JJ, Joubran N, Chelala D, Fares N (2019) VEGF-C attenuates renal damage in salt-sensitive hypertension. J Cell Physiol 234(6):9616–9630. https://doi.org/10.1002/jcp.27648
Huang JL, Woolf AS, Kolatsi-Joannou M, Baluk P, Sandford RN, Peters DJ, McDonald DM, Price KL, Winyard PJ, Long DA (2016) Vascular endothelial growth factor C for polycystic kidney diseases. JASN 27(1):69–77. https://doi.org/10.1681/asn.2014090856
Hasegawa S, Nakano T, Torisu K, Tsuchimoto A, Eriguchi M, Haruyama N, Masutani K, Tsuruya K, Kitazono T (2017) Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab Investig 97(12):1439–1452.https://doi.org/10.1038/labinvest.2017.77
Anders HJ (2016) Of inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol 27(9):2564–2575. https://doi.org/10.1681/asn.2016020177
Ip WK, Medzhitov R (2015) Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation. Nat Commun 6:6931. https://doi.org/10.1038/ncomms7931
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4):728–741. https://doi.org/10.1016/j.cell.2011.10.026
Liu J, Zhuang Y, Wu J, Wu Q, Liu M, Zhao Y, Liu Z, Wang C, Lu L, Meng Y, Lei K, Li X, Wu Q, Leung EL, Guo Z, Liu L, Li T (2022) IKKβ mediates homeostatic function in inflammation via competitively phosphorylating AMPK and IκBα. Acta Pharmaceut Sin B 12(2):651–664. https://doi.org/10.1016/j.apsb.2021.09.012
Drummond GR, Vinh A, Guzik TJ, Sobey CG (2019) Immune mechanisms of hypertension. Nat Rev Immunol 19(8):517–532. https://doi.org/10.1038/s41577-019-0160-5
Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu X, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan SZ (2015) High salt primes a specific activation state of macrophages, M(Na). Cell Res 25(8):893–910. https://doi.org/10.1038/cr.2015.87
Ruggeri Barbaro N, Van Beusecum J, Xiao L, do Carmo L, Pitzer A, Loperena R, Foss JD, Elijovich F, Laffer CL, Montaniel KR, Galindo CL, Chen W, Ao M, Mernaugh RL, Alsouqi A, Ikizler TA, Fogo AB, Moreno H, Zhao S, Davies SS, Harrison DG, Kirabo A (2021) Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc Res 117(5):1358–1371. https://doi.org/10.1093/cvr/cvaa207
Yang GH, Zhou X, Ji WJ, Zeng S, Dong Y, Tian L, Bi Y, Guo ZZ, Gao F, Chen H, Jiang TM, Li YM (2014) Overexpression of VEGF-C attenuates chronic high salt intake-induced left ventricular maladaptive remodeling in spontaneously hypertensive rats. Am J Physiol Heart Circul Physiol 306(4):H598-609. https://doi.org/10.1152/ajpheart.00585.2013
Balasubbramanian D, Baranwal G, Clark MC, Goodlett BL, Mitchell BM, Rutkowski JM (2020) Kidney-specific lymphangiogenesis increases sodium excretion and lowers blood pressure in mice. J Hypertens 38(5):874–885. https://doi.org/10.1097/hjh.0000000000002349
Goodlett BL, Kang CS, Yoo E, Navaneethabalakrishnan S, Balasubbramanian D, Love SE, Sims BM, Avilez DL, Tate W, Chavez DR, Baranwal G, Nabity MB, Rutkowski JM, Kim D, Mitchell BM (2021) A kidney-targeted nanoparticle to augment renal lymphatic density decreases blood pressure in hypertensive mice. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14010084
L. Song, X. Chen, T.A. Swanson, B. LaViolette, J. Pang, T. Cunio, M.W. Nagle, S. Asano, K. Hales, A. Shipstone, H. Sobon, S.D. Al-Harthy, Y. Ahn, S. Kreuser, A. Robertson, C. Ritenour, F. Voigt, M. Boucher, F. Sun, W.C. Sessa, R.J. Roth Flach, Lymphangiogenic therapy prevents cardiac dysfunction by ameliorating inflammation and hypertension. eLife. https://doi.org/10.7554/eLife.58376
D’Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S (2014) VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Investig 124(9):3863–3878. https://doi.org/10.1172/jci72189
Komada T, Muruve DA (2019) The role of inflammasomes in kidney disease. Nat Rev Nephrol 15(8):501–520. https://doi.org/10.1038/s41581-019-0158-z
Zhu X, Li S, Lin Q, Shao X, Wu J, Zhang W, Cai H, Zhou W, Jiang N, Zhang Z, Shen J, Wang Q, Ni Z (2021) αKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol Res 167:105531. https://doi.org/10.1016/j.phrs.2021.105531
Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456(7219):264–268. https://doi.org/10.1038/nature07383
Eisenberg T, Abdellatif M, Zimmermann A, Schroeder S, Pendl T, Harger A, Stekovic S, Schipke J, Magnes C, Schmidt A, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Carmona-Gutierrez D, Pietrocola F, Pieber TR, Sigrist SJ, Linke WA, Mühlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F (2017) Dietary spermidine for lowering high blood pressure. Autophagy 13(4):767–769. https://doi.org/10.1080/15548627.2017.1280225
McCarthy CG, Wenceslau CF, Calmasini FB, Klee NS, Brands MW, Joe B, Webb RC (2019) Reconstitution of autophagy ameliorates vascular function and arterial stiffening in spontaneously hypertensive rats. Am J Physiol Heart Circul Physiol 317(5):H101-h1027. https://doi.org/10.1152/ajpheart.00227.2019
Cadwell K (2016) Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Revi Immunol 16(11):661–675. https://doi.org/10.1038/nri.2016.100
Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12(3):222–230. https://doi.org/10.1038/ni.1980
Starling S (2020) Role for toll-like receptor 9 in muscle AMPK activation. Nat Rev Endocrinol 16(4):197. https://doi.org/10.1038/s41574-020-0337-9
Ma L, Li W, Zhang Y, Qi L, Zhao Q, Li N, Lu Y, Zhang L, Zhou F, Wu Y, He Y, Yu H, He Y, Wei B, Wang H (2022) FLT4/VEGFR3 activates AMPK to coordinate glycometabolic reprogramming with autophagy and inflammasome activation for bacterial elimination. Autophagy 18(6):1385–1400. https://doi.org/10.1080/15548627.2021.1985338
Kim SH, Kim G, Han DH, Lee M, Kim I, Kim B, Kim KH, Song YM, Yoo JE, Wang HJ, Bae SH, Lee YH, Lee BW, Kang ES, Cha BS, Lee MS (2017) Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy 13(10):1767–1781. https://doi.org/10.1080/15548627.2017.1356977
D. Bai, J. Du, X. Bu, W. Cao, T. Sun, J. Zhao, Y. Zhao, N. Lu, ALDOA maintains NLRP3 inflammasome activation by controlling AMPK activation, Autophagy 18(7) (2022) 1673–1693.https://doi.org/10.1080/15548627.2021.1997051
J. Liang, S.H. Shao, Z.X. Xu, B. Hennessy, Z. Ding, M. Larrea, S. Kondo, D.J. Dumont, J.U. Gutterman, C.L. Walker, J.M. Slingerland, G.B. Mills, The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis, Nature cell biology 9(2) (2007) 218–24.https://doi.org/10.1038/ncb1537
S.C. Lin, D.G. Hardie, AMPK: Sensing Glucose as well as Cellular Energy Status, Cell metabolism 27(2) (2018) 299–313.https://doi.org/10.1016/j.cmet.2017.10.009
S.A. Hawley, F.A. Ross, G.J. Gowans, P. Tibarewal, N.R. Leslie, D.G. Hardie, Phosphorylation by Akt within the ST loop of AMPK-α1 down-regulates its activation in tumour cells, The Biochemical journal 459(2) (2014) 275–87.https://doi.org/10.1042/bj20131344
S. Horman, D. Vertommen, R. Heath, D. Neumann, V. Mouton, A. Woods, U. Schlattner, T. Wallimann, D. Carling, L. Hue, M.H. Rider, Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491, The Journal of biological chemistry 281(9) (2006) 5335–40.https://doi.org/10.1074/jbc.M506850200
G. Sahni, Onco-Hypertension: Changing Paradigm of Treating Hypertension in Patients With Cancer, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 41(5) (2023) 958–963.https://doi.org/10.1200/jco.22.01875
S. Kidoguchi, N. Sugano, G. Tokudome, T. Yokoo, Y. Yano, K. Hatake, A. Nishiyama, New Concept of Onco-Hypertension and Future Perspectives, Hypertension (Dallas, Tex. : 1979) 77(1) (2021) 16–27.https://doi.org/10.1161/hypertensionaha.120.16044
van Doorn L, Visser WJ, van Dorst DCH, Mirabito Colafella KM, Koolen SLW, de Mik AVE, Garrelds IM, Bovée DM, de Hoop EO, Bins S, Eskens F, Hoorn EJ, Jan Danser AH, Mathijssen RHJ, Versmissen J (2023) Dietary sodium restriction prevents vascular endothelial growth factor inhibitor-induced hypertension. Br J Cancer 128(2):354–362. https://doi.org/10.1038/s41416-022-02036-6
Hartiala P, Suominen S, Suominen E, Kaartinen I, Kiiski J, Viitanen T, Alitalo K, Saarikko AM (2020) Phase 1 Lymfactin(Ⓡ) Study: Short-term Safety of Combined Adenoviral VEGF-C and Lymph Node Transfer Treatment for Upper Extremity Lymphedema. JPRAS 73(9):1612–1621. https://doi.org/10.1016/j.bjps.2020.05.009
Funding
This work was supported by Joint Guidance Project of Natural Science Foundation of Heilongjiang Province of China [LH2021H027] and Fund of Key Laboratory of Myocardial Ischemia, Ministry of Education [KF202222, KF201703]. Thanks to the staff of The Key Laboratory of Myocardial Ischemia for their help.
Author information
Authors and Affiliations
Contributions
Qiuwen Wu, Sining Hu and Shuo Zhang conceived and designed the research; Qiuwen Wu, Xi Chen, Jiaxin Fu, Chunyu Zhao, Gang Liu and Wenqi Zhao performed the research and acquired the data; Wei Meng, Bin Zhu, Xing Luo, Ying Lv and Sining Hu analyzed and interpreted the data; Qiuwen Wu, Wei Meng and Bin Zhu drafted the original article and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Second Affiliated Hospital of Harbin Medical University (SYDW2022-075).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Q., Meng, W., Zhu, B. et al. VEGFC ameliorates salt-sensitive hypertension and hypertensive nephropathy by inhibiting NLRP3 inflammasome via activating VEGFR3-AMPK dependent autophagy pathway. Cell. Mol. Life Sci. 80, 327 (2023). https://doi.org/10.1007/s00018-023-04978-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04978-3