Abstract
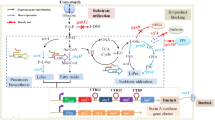
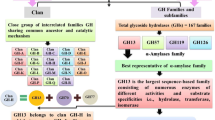
Members of the Bacteroidetes phylum in the human colon deploy an extensive number of proteins to capture and degrade polysaccharides. Operons devoted to glycan breakdown and uptake are termed polysaccharide utilization loci or PUL. The starch utilization system (Sus) is one such PUL and was initially described in Bacteroides thetaiotaomicron (Bt). BtSus is highly conserved across many species, except for its extracellular α-amylase, SusG. In this work, we show that the Bacteroides ovatus (Bo) extracellular α-amylase, BoGH13ASus, is distinguished from SusG in its evolutionary origin and its domain architecture and by being the most prevalent form in Bacteroidetes Sus. BoGH13ASus is the founding member of both a novel subfamily in the glycoside hydrolase family 13, GH13_47, and a novel carbohydrate-binding module, CBM98. The BoGH13ASus CBM98–CBM48–GH13_47 architecture differs from the CBM58 embedded within the GH13_36 of SusG. These domains adopt a distinct spatial orientation and invoke a different association with the outer membrane. The BoCBM98 binding site is required for Bo growth on polysaccharides and optimal enzymatic degradation thereof. Finally, the BoGH13ASus structure features bound Ca2+ and Mn2+ ions, the latter of which is novel for an α-amylase. Little is known about the impact of Mn2+ on gut bacterial function, much less on polysaccharide consumption, but Mn2+ addition to Bt expressing BoGH13ASus specifically enhances growth on starch. Further understanding of bacterial starch degradation signatures will enable more tailored prebiotic and pharmaceutical approaches that increase starch flux to the gut.








Similar content being viewed by others
Availability of data and material
BoGH13ASus native, maltoheptaose and acarbose-bound structures were deposited with the RCSB Protein Data Bank, with PDB IDs of: 8DGE, 8DL1 and 8DL2, respectively. All plasmids, proteins, bacterial strains and other reagents generated in this study will be made freely available to any researcher wishing to use them for non-commercial purposes.
Abbreviations
- AP:
-
Potato amylopectin
- Bo:
-
Bacteroides ovatus
- Bt:
-
Bacteroides thetaiotaomicron
- CBM:
-
Carbohydrate-binding module
- G2:
-
Maltose
- G3:
-
Maltotriose
- G4:
-
Maltotetraose
- G5:
-
Maltopentaose
- G6:
-
Maltohexaose
- G7:
-
Maltoheptaose
- GH:
-
Glycoside hydrolase
- Glc:
-
Glucose
- PS:
-
Potato starch
- PUL:
-
Polysaccharide utilization loci
- Sus:
-
Starch utilization system
References
Reyes A, Semenkovich NP, Whiteson K, Rohwer F, Gordon JI (2012) Going viral: next generation sequencing applied to human gut phage populations. Nat Rev Microbiol 10:607–617. https://doi.org/10.1038/nrmicro2853
Kapitan M, Niemiec MJ, Steimle A, Frick JS, Jacobsen ID (2019) Fungi as part of the microbiota and interactions with intestinal bacteria. Curr Top Microbiol Immunol 422:265–301. https://doi.org/10.1007/82_2018_117
Moissl-Eichinger C, Pausan M, Taffner J, Berg G, Bang C, Schmitz RA (2018) Archaea are interactive components of complex microbiomes. Trends Microbiol 26:70–85. https://doi.org/10.1016/j.tim.2017.07.004
Nayfach S, Shi ZJ, Seshadri R, Pollard KS, Kyrpides NC (2019) New insights from uncultivated genomes of the global human gut microbiome. Nature 568:505–510. https://doi.org/10.1038/s41586-019-1058-x
Wilson AS, Koller KR, Ramaboli MC, Nesengani LT, Ocvirk S, Chen C, Flanagan CA, Sapp FR, Merritt ZT, Bhatti F, Thomas TK, O’Keefe SJD (2020) Diet and the human gut microbiome: an international review. Dig Dis Sci 65:723–740. https://doi.org/10.1007/s10620-020-06112-w
Hansson GC (2020) Mucins and the microbiome. Annu Rev Biochem 89:769–793. https://doi.org/10.1146/annurev-biochem-011520-105053
Koh A, DeVadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Makki K, Deehan EC, Walter J, Bäckhed F (2018) The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–715. https://doi.org/10.1016/j.chom.2018.05.012
McNeil NI (1984) The contribution of the large intestine to energy supplies in man. Am J Clin Nutr 39:338–342. https://doi.org/10.1093/ajcn/39.2.338
Cockburn DW, Koropatkin NM (2016) Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J Mol Biol 428:3230–3252. https://doi.org/10.1016/j.jmb.2016.06.021
Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, Gordon JI (2011) Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol 9:e1001221. https://doi.org/10.1371/journal.pbio.1001221
Martens EC, Chiang HC, Gordon JI (2008) Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4:447–457. https://doi.org/10.1016/j.chom.2008.09.007
McNulty NP, Wu M, Erickson AR, Pan C, Erickson BK, Martens EC, Pudlo NA, Muegge BD, Henrissat B, Hettich RL, Gordon JI (2013) Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol 11:e1001637. https://doi.org/10.1371/journal.pbio.1001637
Brown HA, Koropatkin NM (2021) Host glycan utilization within the Bacteroidetes Sus-like paradigm. Glycobiology 31:697–706. https://doi.org/10.1093/glycob/cwaa054
McKee LS, La Rosa SL, Westereng B, Eijsink VG, Pope PB, Larsbrink J (2021) Polysaccharide degradation by the Bacteroidetes: mechanisms and nomenclature. Environ Microbiol Rep 13:559–581. https://doi.org/10.1111/1758-2229.12980
Martens EC, Koropatkin NM, Smith TJ, Gordon JI (2009) Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem 284:24673–24677. https://doi.org/10.1074/jbc.R109.022848
Grondin JM, Tamura K, Déjean G, Abbott DW, Brumer H (2017) Polysaccharide utilization loci: fueling microbial communities. J Bacteriol 199:e00860-e916. https://doi.org/10.1128/JB.00860-16
Cho KH, Salyers AA (2001) Biochemical analysis of interactions between outer membrane proteins that contribute to starch utilization by Bacteroides thetaiotaomicron. J Bacteriol 183:7224–7230. https://doi.org/10.1128/JB.183.24.7224-7230.2001
D’Elia JN, Salyers AA (1996) Contribution of a neopullulanase, a pullulanase, and an α-glucosidase to growth of Bacteroides thetaiotaomicron on starch. J Bacteriol 178:7173–7179. https://doi.org/10.1128/jb.178.24.7173-7179.1996
Foley MH, Cockburn DW, Koropatkin NM (2016) The Sus operon: a model system for starch uptake by the human gut Bacteroidetes. Cell Mol Life Sci. 73:2603–2617. https://doi.org/10.1007/s00018-016-2242-x
Reeves AR, Wang GR, Salyers AA (1997) Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol 179:643–649. https://doi.org/10.1128/jb.179.3.643-649.1997a
Shipman JA, Cho KH, Siegel HA, Salyers AA (1999) Physiological characterization of SusG, an outer membrane protein essential for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol 181:7206–7211. https://doi.org/10.1128/JB.181.23.7206-7211.1999
Koropatkin NM, Smith TJ (2010) SusG: a unique cell-membrane-associated α-amylase from a prominent human gut symbiont targets complex starch molecules. Structure 18:200–215. https://doi.org/10.1016/j.str.2009.12.010
Cameron EA, Kwiatkowski KJ, Lee BH, Hamaker BR, Koropatkin NM, Martens EC (2014) Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis. MBio 5:e01441-e1514. https://doi.org/10.1128/mBio.01441-14
Foley MH, Martens EC, Koropatkin NM (2018) SusE facilitates starch uptake independent of starch binding in B. thetaiotaomicron. Mol Microbiol 108:551–566. https://doi.org/10.1111/mmi.13949
Tuson HH, Foley MH, Koropatkin NM, Biteen JS (2018) The starch utilization system assembles around stationary starch-binding proteins. Biophys J 14:242–250. https://doi.org/10.1016/j.bpj.2017.12.015
Karunatilaka KS, Cameron EA, Martens EC, Koropatkin NM, Biteen JS (2014) Superresolution imaging captures carbohydrate utilization dynamics in human gut symbionts. MBio 5:e02172-14. https://doi.org/10.1128/mBio.02172-14
Geffroy L, Brown HA, DeVeaux AL, Koropatkin NM, Biteen JS (2022) Single-molecule dynamics of surface lipoproteins in Bacteroides indicate similarities and cooperativity. Biophys J 121:4644–4655. https://doi.org/10.1016/j.bpj.2022.10.024
Drula E, Garron ML, Dogan S, Lombard V, Henrissat B, Terrapon N (2022) The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res 50:D571–D577. https://doi.org/10.1093/nar/gkab1045
Flint HJ, Whitehead TR, Martin JC, Gasparic A (1997) Interrupted catalytic domain structures in xylanases from two distantly related strains of Prevotella ruminicola. Biochem Biophys Acta 1337:161–165. https://doi.org/10.1016/s0167-4838(96)00213-0
Zhang M, Chekan JR, Dodd D, Hong PY, Radlinski L, Revindran V, Nair SK, Mackie RI, Cann I (2014) Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc Natl Acad Sci U S A 111:E3708–E3717. https://doi.org/10.1073/pnas.1406156111
Machovič M, Svensson B, MacGregor EA, Janeček Š (2005) A new clan of CBM families based on bioinformatics of starch-binding domains from families CBM20 and CBM21. FEBS J 272:5497–5513. https://doi.org/10.1111/j.1742-4658.2005.04942.x
Lapébie P, Lombard V, Drula E, Terrapon N, Henrissat B (2019) Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat Commun 10:2043. https://doi.org/10.1038/s41467-019-10068-5
MacGregor EA, Janeček Š, Svensson B (2001) Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochim Biophys Acta 1546:1–20. https://doi.org/10.1016/s0167-4838(00)00302-2
Arnal G, Cockburn DW, Brumer H, Koropatkin NM (2018) Structural basis for the flexible recognition of α-glucan substrates by Bacteroides thetaiotaomicron SusG. Protein Sci 27:1093–1101. https://doi.org/10.1002/pro.3410
Davies GJ, Wilson KS, Henrissat B (1997) Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochem J. https://doi.org/10.1042/bj3210557
Gilbert RG, Wu AC, Sullivan MA, Sumarriva GE, Ersch N, Hasjim J (2013) Improving human health through understanding the complex structure of glucose polymers. Anal Bioanal Chem 405:8969–8980. https://doi.org/10.1007/s00216-013-7129-1
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos
The PyMOL molecular graphics system, Version 2.5.2 Schrödinger, LLC
Cockburn DW, Cerqueira FM, Bahr C, Koropatkin NM (2020) The structures of the GH13_36 amylases from Eubacterium rectale and Ruminococcus bromii reveal subsite architectures that favor maltose production. Amylase 4:24–44. https://doi.org/10.1515/amylase-2020-0003
Kagawa M, Fujimoto Z, Momma M, Takase K, Mizuno H (2003) Crystal structure of Bacillus subtilis alpha-amylase in complex with acarbose. J Bacteriol 185:6981–6984. https://doi.org/10.1128/JB.185.23.6981-6984.2003
Abe A, Tonozuka T, Sakano Y, Kamitori S (2004) Complex structures of Thermoactinomyces vulgaris R-47 α-amylase 1 with malto-oligosaccharides demonstrate the role of domain N acting as a starch-binding domain. J Mol Biol 335:811–822. https://doi.org/10.1016/j.jmb.2003.10.078
Holm L, Sander C (1995) Dali: a network tool for protein structure comparison. Trends Biochem Sci 20:478–480. https://doi.org/10.1016/s0968-0004(00)89105-7
Chai KP, Othman NFB, Teh AH, Ho KL, Chan KG, Shamsir MS, Goh KM, Ng CL (2016) Crystal structure of Anoxybacillus α-amylase provides insights into maltose binding of a new glycosyl hydrolase subclass. Sci Rep 6:23126. https://doi.org/10.1038/srep23126
Mok SC, Teh AH, Saito JA, Najimudin N, Alam M (2013) Crystal structure of a compact α-amylase from Geobacillus thermoleovorans. Enzyme Microb Technol 53:46–54. https://doi.org/10.1016/j.enzmictec.2013.03.009
Janeček Š, Kuchtová A, Petrovičová S (2015) A novel GH13 subfamily of α-amylases with a pair of tryptophans in the helix α3 of the catalytic TIM-barrel, the LPDlx signature in the conserved sequence region V and a conserved aromatic motif at the C-terminus. Biologia 70:1284–1294. https://doi.org/10.1515/biolog-2015-0165
Armenteros JJA, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37:420–423. https://doi.org/10.1038/s41587-019-0036-z
Valguarnera E, Scott NE, Azimzadeh P, Feldman MF (2018) Surface exposure and packing of lipoproteins into outer membrane vesicles are coupled processes in Bacteroides. mSphere. 3:e00559-e618. https://doi.org/10.1128/mSphere.00559-18
Boel E, Brady L, Brzozowski AM, Derewenda Z, Dodson GG, Jensen VJ, Petersen SB, Swift H, Thim L, Woldike HF (1990) Calcium binding in alpha-amylases: an X-ray diffraction study at 2.1-Å resolution of two enzymes from Aspergillus. Biochemistry 29:6244–6249. https://doi.org/10.1021/bi00478a019
Kirberger M, Yang JJ (2013) Calcium-binding protein site types. In: Kretsinger RG, Uversky VN, Permyakov EA (eds) Encylcopedia of metalloproteins. Springer, New York, pp 511–521
Khrustalev VV, Barkovsky EV, Khrustaleva TA (2016) Magnesium and manganese binding sites on proteins have the same predominant motif of secondary structure. J Theor Biol 395:174–185. https://doi.org/10.1016/j.jtbi.2016.02.006
Harding MM (2000) Geometry of metal-ligand interactions in proteins. Acta Crystallogr D Biol Crystallogr 57:401–411. https://doi.org/10.1107/s0907444900019168
Gucwa M, Lenkiewicz J, Zheng H, Cymborowski M, Cooper DR, Murzyn K, Minor W (2022) CMM—an enhanced platform for interactive validation of metal binding sites. Protein Sci. https://doi.org/10.1002/pro.4525
Nonaka T, Fujihashi M, Kita A, Hagihara H, Ozaki K, Ito S, Miki K (2003) Crystal structure of calcium-free alpha-amylase from Bacillus sp. Strain KSM-K38 (AmyK38) and its sodium ion binding sites. J Biol Chem 278:24818–24824. https://doi.org/10.1074/jbc.M212763200
Machius M, Declerck N, Huber R, Wiegand G (1998) Activation of Bacillus licheniformis alpha-amylase through a disorder–>order transition of the substrate-binding site mediated by a calcium–sodium–calcium metal triad. Structure 6:281–292. https://doi.org/10.1016/s0969-2126(98)00032-x
Linden A, Mayans O, Meyer-Klaucke W, Antranikian G, Wilmanns M (2003) Differential regulation of a hyperthermophilic α-amylase with a novel (Ca, Zn) two-metal center by zinc. J Biol Chem. https://doi.org/10.1074/jbc.M211339200
Kamitori S, Kondo S, Okuyama K, Yokota T, Shimura Y, Tonozuka T, Sakano Y (1999) Crystal structure of Thermoactinomyces vulgaris R-47 alpha-amylase II (TVAII) hydrolyzing cyclodextrins and pullulan at 2.6 Å resolution. J Mol Biol 287:907–921. https://doi.org/10.1006/jmbi.1999.2647
Kadziola A, Abe J, Svensson B, Haser R (1994) Crystal and molecular structure of barley alpha-amylase. J Mol Biol 239:104–121. https://doi.org/10.1006/jmbi.1994.1354
Hayashi M, Suzuki R, Colleoni C, Ball SG, Fujita N, Suzuki E (2017) Bound substrate in the structure of cyanobacterial branching enzyme supports a new mechanistic model. J Biol Chem 292:5465–5475. https://doi.org/10.1074/jbc.M116.755629
Tung JY, Chang MD, Chou WI, Liu YY, Yeh YH, Chang FY, Lin SC, Qiu ZL, Sun YJ (2008) Crystal structures of the starch-binding domain from Rhizopus oryzae glucoamylase reveal a polysaccharide-binding path. Biochem J 416:27–36. https://doi.org/10.1042/BJ20080580
Cockburn D, Nielsen MM, Christiansen C, Andersen JM, Rannes JB, Blennow A, Svensson B (2015) Surface binding sites in amylase have distinct roles in recognition of starch structure motifs and degradation. Int J Biol Macromol 75:338–345. https://doi.org/10.1016/j.ijbiomac.2015.01.054
Shallom D, Belakhov V, Solomon D, Shoham G, Baasov T, Shoham Y (2002) Detailed kinetic analysis and identification of the nucleophile in alpha-l-arabinofuranosidase from Geobacillus stearothermophilus T-6, a family 51 glycoside hydrolase. J Biol Chem 277:43667–43673. https://doi.org/10.1074/jbc.M208285200
Park KH, Kim TJ, Cheong TK, Kim JW, Oh BH, Svensson B (2000) Structure, specificity and function of cyclomaltodextrinase, a multispecific enzyme of the alpha-amylase family. Biochim Biophys Acta 1478:165–185. https://doi.org/10.1016/s0167-4838(00)00041-8
Kim TJ, Kim MJ, Kim BC, Kim JC, Cheong TK, Kim JW, Park KH (1999) Modes of action of acarbose hydrolysis and transglycosylation catalyzed by a thermostable maltogenic amylase, the gene for which was cloned from a Thermus strain. Appl Environ Microbiol 65:1644–1651. https://doi.org/10.1128/AEM.65.4.1644-1651.1999
Horváthová V, Janeček Š, Šturdík E (2001) Amylolytic enzymes: molecular aspects of their properties. Gen Physiol Biophys 20:7–32
McIver LA, Preuss CV, Tripp J (2022) Acarbose. In: StatPearls. StatPearls Publishing, Treasure Island
Ferey-Roux G, Perrier J, Forest E, Marchis-Mouren G, Puigserver A, Santimone M (1998) The human pancreatic alpha-amylase isoforms: isolation, structural studies and kinetics of inhibition by acarbose. Biochim Biophys Acta 1388:10–20. https://doi.org/10.1016/s0167-4838(98)00147-2
Lee BH, Eskandari R, Jones K, Reddy KR, Quezada-Calvillo R, Nichols BL, Rose DR, Hamaker BR, Pinto BM (2012) Modulation of starch digestion for slow glucose release through “toggling” of activities of mucosal alpha-glucosidases. J Biol Chem 287:31929–31938. https://doi.org/10.1074/jbc.M112.351858
Li C, Begum A, Numao S, Park KH, Withers SG, Brayer GD (2005) Acarbose rearrangement mechanism implied by the kinetic and structural analysis of human pancreatic alpha-amylase in complex with analogues and their elongated counterparts. Biochemistry 44:3347–3357. https://doi.org/10.1021/bi048334e
Brzozowski AM, Davies GJ (1997) Structure of the Aspergillus oryzae alpha-amylase complexed with the inhibitor acarbose at 2.0 Å resolution. Biochemistry 36:10837–10845. https://doi.org/10.1021/bi970539i
Kadziola A, Søgaard M, Svensson B, Haser R (1998) Molecular structure of a barley alpha-amylase-inhibitor complex: implications for starch binding and catalysis. J Mol Biol 278:205–217. https://doi.org/10.1006/jmbi.1998.1683
Gilles C, Astier JP, Marchis-Mouren G, Cambillau C, Payan F (1996) Crystal structure of pig pancreatic alpha-amylase isoenzyme II, in complex with the carbohydrate inhibitor acarbose. Eur J Biochem 238:561–569. https://doi.org/10.1111/j.1432-1033.1996.0561z.x
Abbott DW, Boraston AB (2012) Quantitative approaches to the analysis of carbohydrate-binding module function. Methods Enzymol 510:211–231. https://doi.org/10.1016/B978-0-12-415931-0.00011-2
Cockburn D, Wilkens C, Svensson B (2017) Affinity electrophoresis for analysis of catalytic module-carbohydrate interactions. Methods Mol Biol 1588:119–127. https://doi.org/10.1007/978-1-4939-6899-2_9
Degnan PH, Barry NA, Mok KC, Taga ME, Goodman AL (2014) Human gut microbes use multiple transporters to distinguish vitamin B12 analogs and compete in the gut. Cell Host Microbe 15:47–57. https://doi.org/10.1016/j.chom.2013.12.007
Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, Baslé A, Cartmell A, Terrapon N, Stott K, Lowe EC, McLean R, Shearer K, Schückel J, Venditto I, Ralet MC, Henrissat B, Martens EC, Mosimann SC, Abbott DW, Gilbert HJ (2018) Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat Microbiol 3:210–219. https://doi.org/10.1038/s41564-017-0079-1
Bøger M, Hekelaar J, van Leeuwen SS, Dijkhuizen L, Lammerts van Bueren A (2019) Structural and functional characterization of a family GH53 beta-1,4-galactanase from Bacteroides thetaiotaomicron that facilitates degradation of prebiotic galactooligosaccharides. J Struct Biol 205:1–10. https://doi.org/10.1016/j.jsb.2018.12.002
Kehres DG, Maguire ME (2003) Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27:263–290. https://doi.org/10.1016/S0168-6445(03)00052-4
Terrapon N, Lombard V, Drula É, Lapébie P, Al-Masaudi S, Gilbert HJ, Henrissat B (2018) PULDB: the expanded database of polysaccharide utilization loci. Nucleic Acids Res 46:D677–D683. https://doi.org/10.1093/nar/gkx1022
Stam MR, Danchin EJG, Rancurel C, Coutinho PM, Henrissat B (2006) Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of alpha-amylase-related proteins. Protein Eng Des Sel 19:555–562. https://doi.org/10.1093/protein/gzl044
Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, Ben-Tal N (2016) ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res 44:W344–W350. https://doi.org/10.1093/nar/gkw408
Celniker G, Nimrod G, Ashkenazy H, Glaser F, Martz E, Mayrose I, Pupko T, Ben-Tal N (2013) ConSurf: using evolutionary data to raise testable hypotheses about protein function. Isr J Chem 53:199–206. https://doi.org/10.1002/ijch.201200096
Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N (2010) ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res 38:W529–W533. https://doi.org/10.1093/nar/gkq399
de Jonge PA, von Meijenfeldt FAB, van Rooijen LE, Brouns SJJ, Dutilh BE (2019) Evolution of BACON domain tandem repeats in crAssphage and novel gut bacteriophage lineages. Viruses. https://doi.org/10.3390/v11121085
Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, Klinter S, Pudlo NA, Urs K, Koropatkin NM, Creagh AL, Haynes CA, Kelly AG, Cederholm SN, Davies GJ, Martens EC, Brumer H (2014) A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 506:498–502. https://doi.org/10.1038/nature12907
Lasica AM, Ksiazek M, Madej M, Potempa J (2017) The type IX secretion system (T9SS): highlights and recent insights into its structure and function. Front Cell Infect Microbiol 7:215. https://doi.org/10.3389/fcimb.2017.00215
Joglekar P, Sonnenburg ED, Higginbottom SK, Earle KA, Morland C, Shapiro-Ward S, Bolam DN, Sonnenburg JL (2018) Genetic variation of the SusC/SusD homologs from a polysaccharide utilization locus underlies divergent fructan specificities and functional adaptation in Bacteroides thetaiotaomicron strains. mSphere. https://doi.org/10.1128/mSphereDirect.00185-18
Déjean G, Tamura K, Cabrera A, Jain N, Pudlo NA, Pereira G, Viborg AH, Van Petegem F, Martens EC, Brumer H (2020) Synergy between cell surface glycosidases and glycan-binding proteins dictates the utilization of specific beta(1,3)-glucans by human gut Bacteroides. MBio. https://doi.org/10.1128/mBio.00095-20
Tamura K, Déjean G, Van Petegem F, Brumer H (2021) Distinct protein architectures mediate species-specific beta-glucan binding and metabolism in the human gut microbiota. J Biol Chem 296:100415. https://doi.org/10.1016/j.jbc.2021.100415
Tuncil YE, Xiao Y, Porter NT, Reuhs BL, Martens EC, Hamaker BR (2017) Reciprocal prioritization to dietary glycans by gut bacteria in a competitive environment promotes stable coexistence. MBio 8:e01068-e1117. https://doi.org/10.1128/mBio.01068-17
Montanier C, Lammerts van Bueren A, Dumon C, Flint JE, Correia MA, Prates JA, Firbank SJ, Lewis RJ, Grondin GG, Ghinet MG, Gloster TM, Herve C, Knox JP, Talbot BG, Turkenburg JP, Kerovuo J, Brzezinski R, Fontes CMGA, Davies GJ, Boraston AB, Gilbert HJ (2009) Evidence that family 35 carbohydrate binding modules display conserved specificity but divergent function. Proc Natl Acad Sci U S A 106:3065–3070. https://doi.org/10.1073/pnas.0808972106
Ezer A, Matalon E, Jindou S, Borovok I, Atamna N, Yu Z, Morrison M, Bayer EA, Lamed R (2008) Cell surface enzyme attachment is mediated by family 37 carbohydrate-binding modules, unique to Ruminococcus albus. J Bacteriol 190:8220–8222. https://doi.org/10.1128/JB.00609-08
Møller MS, Henriksen A, Svensson B (2016) Structure and function of alpha-glucan debranching enzymes. Cell Mol Life Sci 73:2619–2641. https://doi.org/10.1007/s00018-016-2241-y
Feng L, Fawaz R, Hovde S, Gilbert L, Chiou J, Geiger JH (2015) Crystal structures of Escherichia coli branching enzyme in complex with linear oligosaccharides. Biochemistry 54:6207–6218. https://doi.org/10.1021/acs.biochem.5b00228
Janeček Š, Svensson B, MacGregor EA (2011) Structural and evolutionary aspects of two families of non-catalytic domains present in starch and glycogen binding proteins from microbes, plants and animals. Enzyme Microb Technol 49:429–440. https://doi.org/10.1016/j.enzmictec.2011.07.002
White JBR, Silale A, Feasey M, Heunis T, Zhu Y, Zheng H, Gajbhiye A, Firbank S, Baslé A, Trost M, Bolam DN, van den Berg B, Ranson NA (2023) Outer membrane utilisomes mediate glycan uptake in gut Bacteroidetes. Nature. 618:583–589. https://doi.org/10.1038/s41586-023-06146-w
Thompson J, Hess S, Pikis A (2004) Genes malh and pagl of Clostridium acetobutylicum ATCC 824 encode NAD+- and Mn2+-dependent phospho-alpha-glucosidase(s). J Biol Chem 279:1553–1561. https://doi.org/10.1074/jbc.M310733200
Raasch C, Streit W, Schanzer J, Bibel M, Gosslar U, Liebl W (2000) Thermotoga maritima AglA, an extremely thermostable NAD+-, Mn2+-, and thiol-dependent alpha-glucosidase. Extremophiles 4:189–200. https://doi.org/10.1007/pl00010711
Nielsen FH (2012) Manganese, molybdenum, boron, chromium, and other trace elements. In: Erdman JW Jr, Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition, 10th edn. Wiley-Blackwell, Ames, pp 586–607
Guiberson ER, Good CJ, Wexler AG, Skaar EP, Spraggins JM, Caprioli RM (2022) Multimodal imaging mass spectrometry of murine gastrointestinal tract with retained luminal content. J Am Soc Mass Spectrom 33:1073–1076. https://doi.org/10.1021/jasms.1c00360
Davis CD, Zech L, Greger JL (1993) Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc Soc Exp Biol Med 202:103–108. https://doi.org/10.3181/00379727-202-43518
Yu X, Han J, Li H, Zhang Y, Feng J (2018) The effect of enzymes on release of trace elements in feedstuffs based on in vitro digestion model for monogastric livestock. J Anim Sci Biotechnol 9:73. https://doi.org/10.1186/s40104-018-0289-2
Pajarillo EAB, Lee E, Kang DK (2021) Trace metals and animal health: interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim Nutr 7:750–761. https://doi.org/10.1016/j.aninu.2021.03.005
Lopez CA, Skaar EP (2018) The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe 23:737–748. https://doi.org/10.1016/j.chom.2018.05.008
Damo S, Chazin WJ, Skaar EP, Kehl-Fie TE (2012) Inhibition of bacterial superoxide defense: a new front in the struggle between host and pathogen. Virulence 3:325–328. https://doi.org/10.4161/viru.19635
Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio SP, Rathi SG, Mastroianni JR, Edwards RA, Jacobo CM, Cerasi M, Battistoni A, Ouellette AJ, Goulding CW, Chazin WJ, Skaar EP, Raffatellu M (2016) Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe 19:814–825. https://doi.org/10.1016/j.chom.2016.05.005
DeMartino P, Cockburn DW (2020) Resistant starch: impact on the gut microbiome and health. Curr Opin Biotechnol 61:66–71. https://doi.org/10.1016/j.copbio.2019.10.008
Cerqueira FM, Photenhauer AL, Pollet RM, Brown HA, Koropatkin NM (2020) Starch digestion by gut bacteria: crowdsourcing for carbs. Trends Microbiol 28:95–108. https://doi.org/10.1016/j.tim.2019.09.004
Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, Zhang D, Feng Q, Xie X, Hong J, Ren H, Liu W, Ma J, Su Q, Zhang H, Yang J, Wang X, Zhao X, Gu W, Bi Y, Peng Y, Xu X, Xia H, Li F, Xu X, Yang H, Xu G, Madsen L, Kristiansen K, Ning G, Wang W (2017) Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun 8:1785. https://doi.org/10.1038/s41467-017-01682-2
Pudlo NA, Urs K, Crawford R, Pirani A, Atherly T, Jimenez R, Terrapon N, Henrissat B, Peterson D, Ziemer C, Snitkin E, Martens EC (2022) Phenotypic and genomic diversification in complex carbohydrate-degrading human gut bacteria. mSystems. 7:e0094721. https://doi.org/10.1128/msystems.00947-21
Holdeman LV, Moore WEC (eds) (1977) Anaerobe laboratory manual, 4th edn. Virginia Polytechnic Institute and State University, Blacksburg
Koropatkin NM, Martens EC, Gordon JI, Smith TJ (2008) Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16:1105–1115. https://doi.org/10.1016/j.str.2008.03.017
Winter G, Lobley CM, Prince SM (2013) Decision making in xia2. Acta Crystallogr D Biol Crystallogr 69:1260–1273. https://doi.org/10.1107/S0907444913015308
Kabsch W (2010) XDS. Acta Crystallogr D Biol Crystallogr 66:125–132. https://doi.org/10.1107/S0907444909047337
Long F, Vagin AA, Young P, Murshudov GN (2008) BALBES: a molecular-replacement pipeline. Acta Crystallogr D Biol Crystallogr 64:125–132. https://doi.org/10.1107/S0907444907050172
Terwilliger TC, Grosse-Kunstleve RW, Afonine PV, Moriarty NW, Zwart PH, Hung LW, Read RJ, Adams PD (2008) Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr D Biol Crystallogr 64:61–69. https://doi.org/10.1107/S090744490705024X
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. https://doi.org/10.1107/S0907444904019158
Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Žídek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S (2022) AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439–D444. https://doi.org/10.1093/nar/gkab1061
Zhai X, Wu K, Ji R, Zhao Y, Lu J, Yu Z, Xu X, Huang J (2022) Structure and function insight of the alpha-glucosidase QsGH13 from Qipengyuania seohaensis sp. Sw-135. Front Microbiol 13:849585. https://doi.org/10.3389/fmicb.2022.849585
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. https://doi.org/10.1107/S0907444910007493
Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67:355–367. https://doi.org/10.1107/S0907444911001314
Beilsten-Edmands J, Winter G, Gildea R, Parkhurst J, Waterman D, Evans G (2020) Scaling diffraction data in the DIALS software package: algorithms and new approaches for multi-crystal scaling. Acta Crystallogr D Struct Biol 76:385–399. https://doi.org/10.1107/S2059798320003198
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) Phaser crystallographic software. J Appl Crystallogr 40:658–674. https://doi.org/10.1107/S0021889807021206
Liebschner D, Afonine PV, Baker ML, Bunkoczi G, Chen VB, Croll TI, Hintze B, Hung LW, Jain S, McCoy AJ, Moriarty NW, Oeffner RD, Poon BK, Prisant MG, Read RJ, Richardson JS, Richardson DC, Sammito MD, Sobolev OV, Stockwell DH, Terwilliger TC, Urzhumtsev AG, Videau LL, Williams CJ, Adams PD (2019) Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol 75:861–877. https://doi.org/10.1107/S2059798319011471
Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. https://doi.org/10.1107/S0907444909052925
Evans PR, Murshudov GN (2013) How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 69:1204–1214. https://doi.org/10.1107/S0907444913000061
Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58:1948–1954. https://doi.org/10.1107/s0907444902016657
Agirre J, Iglesias-Fernández J, Rovira C, Davies GJ, Wilson KS, Cowtan KD (2015) Privateer: software for the conformational validation of carbohydrate structures. Nat Struct Mol Biol 22:833–834. https://doi.org/10.1038/nsmb.3115
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
McKee LS (2017) Measuring enzyme kinetics of glycoside hydrolases using the 3,5-dinitrosalicylic acid assay. Methods Mol Biol 1588:27–36. https://doi.org/10.1007/978-1-4939-6899-2_3
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Madeira F, Pearce M, Tivey ARN, Basutkar P, Lee J, Edbali O, Madhusoodanan N, Kolesnikov A, Lopez R (2022) Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res 50:W276–W279. https://doi.org/10.1093/nar/gkac240
Cameron EA, Maynard MA, Smith CJ, Smith TJ, Koropatkin NM, Martens EC (2012) Multidomain carbohydrate-binding proteins involved in Bacteroides thetaiotaomicron starch metabolism. J Biol Chem 287:34614–34625. https://doi.org/10.1074/jbc.M112.397380
Needleman SB, Wunsch CD (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48:443–453. https://doi.org/10.1016/0022-2836(70)90057-4
DNASTAR, MegAlign Pro ®. Version 17.3.058. Madison, WI
Lemoine F, Correia D, Lefort V, Doppelt-Azeroual O, Mareuil F, Cohen-Boulakia S, Gascuel O (2019) NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res 47:W260–W265. https://doi.org/10.1093/nar/gkz303
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Criscuolo A, Gribaldo S (2010) BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol 10:210. https://doi.org/10.1186/1471-2148-10-210
Lefort V, Desper R, Gascuel O (2015) FastME 2.0: a comprehensive, accurate, and fast distance-based phylogeny inference program. Mol Biol Evol 32:2798–2800. https://doi.org/10.1093/molbev/msv150
Junier T, Zdobnov EM (2010) The Newick utilities: high-throughput phylogenetic tree processing in the unix shell. Bioinformatics 26:1669–1670. https://doi.org/10.1093/bioinformatics/btq243
Letunic I, Bork P (2021) Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. https://doi.org/10.1093/nar/gkab301
Sorimachi K, Le Gal-Coëffet MF, Williamson G, Archer DB, Williamson MP (1997) Solution structure of the granular starch binding domain of Aspergillus niger glucoamylase bound to beta-cyclodextrin. Structure 5:647–661. https://doi.org/10.1016/s0969-2126(97)00220-7
Møller MS, Abou Hachem M, Svensson B, Henriksen A (2012) Structure of the starch-debranching enzyme barley limit dextrinase reveals homology of the N-terminal domain to CBM21. Acta Crystallogr Sect F Struct Biol Cryst Commun 68:1008–1012. https://doi.org/10.1107/S1744309112031004
Marty MT, Baldwin AJ, Marklund EG, Hochberg GK, Benesch JL, Robinson CV (2015) Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal Chem 87:4370–4376. https://doi.org/10.1021/acs.analchem.5b00140
Mareček F, Janeček Š (2022) A novel subfamily GH13_46 of the α-amylase family GH13 represented by the cyclomaltodextrinase from Flavobacterium sp. No 92. Molecules 27:8735. https://doi.org/10.3390/molecules27248735
Acknowledgements
We thank members of the Koropatkin, Martens and Ruotolo laboratories for helpful feedback on this work.
Funding
This work was supported by the following National Institutes of Health (NIH) grants from the National Institute of General Medical Sciences (NIGMS): R01-GM118475 to N.M.K. and R01-GM095832 to B.T.R. H.A.B was supported by an NIH Ruth L. Kirschstein Postdoctoral National Research Service Award F32-AT011278 from the National Center for Complementary and Integrative Health. The QE UMHR work was supported by the University of Michigan Biosciences Initiative. Use of the Pilatus 3 1 M detector was provided by grant 1S10OD018090-01 from NIGMS at the NIH. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (grant no.: 085P1000817). N.T. and M.B. were supported by the Agence National de la Recherche [grant number ANR-20-CE20-0022].
Author information
Authors and Affiliations
Contributions
HAB cloned, expressed and purified protein constructs as well as performed crystallography experiments, X-ray data collection and structural model refinement, cloned and generated all variant Bt and Bo strains and performed bacterial growth experiments, TLC, ITC, Native PAGE, Western blot, enzyme kinetics, protein sequence analysis, and wrote and edited the original manuscript. ALD cloned, expressed and purified protein constructs, performed TLC and crystallography experiments and did structural model refinement, as well as collected ITC data. BRJ collected and analyzed native MS and ICP-MS data. ALP performed ITC experiments. MB performed bioinformatics analyses of CBM98 and GH13_47. REB assisted with ICP-MS data collection. ZW collected and processed X-ray data. BTR helped direct research and analyzed MS data. NT performed bioinformatics analyses of CBM98 and GH13_47, created the CBM98 family and GH13_47 subfamily within CAZy and analyzed their distribution in the private CAZy database. HAB, BLJ, MB and NT were responsible for data visualization. NMK helped design and direct research and wrote the original manuscript. All authors provided input on the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown, H.A., DeVeaux, A.L., Juliano, B.R. et al. BoGH13ASus from Bacteroides ovatus represents a novel α-amylase used for Bacteroides starch breakdown in the human gut. Cell. Mol. Life Sci. 80, 232 (2023). https://doi.org/10.1007/s00018-023-04812-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04812-w