Abstract
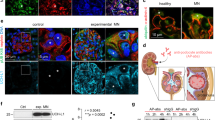
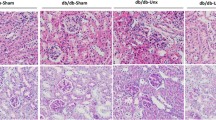
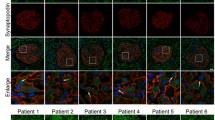
Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) is a unique component of the ubiquitin–proteasome system (UPS), which has multiple activities in maintaining intracellular ubiquitin levels. We previously reported the aberrant low expression of UCHL1 in podocytes of non-immune complex-mediated glomerulonephritis, and recent studies indicate that anti-UCHL1 antibody was responsible for the refractory minimal change disease (MCD), but the specific effect of UCHL1 to the podocytopathy has not been determined. Therefore, we generated podocyte-specific UCHL1 gene knockout (UCHL1cre/cre) rats model. Podocyte-specific UCHL1 knockout rats exhibited severe kidney damage, including segmental/global glomerulosclerosis, kidney function damage and severe proteinuria, compared with littermate control. Subsequently, by carrying out mass spectrometry analysis of isolated glomeruli of rats, abnormal protein accumulation of ECM-receptor Interaction was found in UCHL1cre/cre rats. Mechanistic studies in vivo and in vitro revealed that aberrant protein accumulation after UCHL1 deficiency induced endoplasmic reticulum (ER) stress, unfolded protein reaction (UPR) to reduce the protein level of podocyte skeleton proteins, and CHOP mediated apoptosis as well, which related to the dysfunction of the ubiquitin–proteasome system with decreased free monomeric ubiquitin level, thereby affecting protein ubiquitination and degradation. In addition, inhibition of ER stress by 4-PBA could attenuate the degree of ER stress and podocyte dysfunction. Our study indicates that UCHL1 is a potential target for preventing podocytes injury in some non-immune complex-mediated glomerulopathy.







Similar content being viewed by others
Availability of data and material
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Shankland SJ (2006) The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int 69(12):2131–2147. https://doi.org/10.1038/sj.ki.5000410
Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89(6):1221–1230. https://doi.org/10.1016/j.kint.2016.01.012
Bishop P, Rocca D, Henley JM (2016) Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction. Biochem J 473(16):2453–2462. https://doi.org/10.1042/BCJ20160082
Day IN, Thompson RJ (2010) UCHL1 (PGP 9.5): neuronal biomarker and ubiquitin system protein. Prog Neurobiol 90(3):327–362. https://doi.org/10.1016/j.pneurobio.2009.10.020
Bett JS, Ritorto MS, Ewan R et al (2015) Ubiquitin C-terminal hydrolases cleave isopeptide- and peptide-linked ubiquitin from structured proteins but do not edit ubiquitin homopolymers. Biochem J 466(3):489–498. https://doi.org/10.1042/BJ20141349
Liu Y, Fallon L, Lashuel HA et al (2002) The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell 111(2):209–218. https://doi.org/10.1016/s0092-8674(02)01012-7
Osaka H, Wang YL, Takada K et al (2003) Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet 12(16):1945–1958. https://doi.org/10.1093/hmg/ddg211
Cartier AE, Djakovic SN, Salehi A et al (2009) Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J Neurosci 29(24):7857–7868. https://doi.org/10.1523/JNEUROSCI.1817-09.2009
Erpapazoglou Z, Walker O, Haguenauer-Tsapis R (2014) Versatile roles of k63-linked ubiquitin chains in trafficking. Cells 3(4):1027–1088. https://doi.org/10.3390/cells3041027
Susor A, Liskova L, Toralova T et al (2010) Role of ubiquitin C-terminal hydrolase-L1 in antipolyspermy defense of mammalian oocytes. Biol Reprod 82(6):1151–1161. https://doi.org/10.1095/biolreprod.109.081547
Wang YL, Takeda A, Osaka H et al (2004) Accumulation of beta- and gamma-synucleins in the ubiquitin carboxyl-terminal hydrolase L1-deficient gad mouse. Brain Res 1019(1–2):1–9. https://doi.org/10.1016/j.brainres.2004.05.023
Lohmann F, Sachs M, Meyer TN et al (1842) (2014) UCH-L1 induces podocyte hypertrophy in membranous nephropathy by protein accumulation. Biochim Biophys Acta 7:945–958. https://doi.org/10.1016/j.bbadis.2014.02.011
Liu Y, Wu J, Wu H et al (2009) UCH-L1 expression of podocytes in diseased glomeruli and in vitro. J Pathol 217(5):642–653. https://doi.org/10.1002/path.2511
Radon V, Czesla M, Reichelt J et al (2018) Ubiquitin C-Terminal Hydrolase L1 is required for regulated protein degradation through the ubiquitin proteasome system in kidney. Kidney Int 93(1):110–127. https://doi.org/10.1016/j.kint.2017.05.016
Jamin A, Berthelot L, Couderc A et al (2018) Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun 89:149–161. https://doi.org/10.1016/j.jaut.2017.12.014
Chen G, Nagasawa R, Imasawa T et al (1999) Identification of soluble interleukin-4 receptor in rat glomerular epithelial cells(1). Biochim Biophys Acta 1452(1):79–88. https://doi.org/10.1016/s0167-4889(99)00117-2
Sanden SK, Wiggins JE, Goyal M et al (2003) Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms’ tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol 14(10):2484–2493. https://doi.org/10.1097/01.asn.0000089829.45296.7c
Frakes AE, Dillin A (2017) The UPR(ER): sensor and coordinator of organismal homeostasis. Mol Cell 66(6):761–771. https://doi.org/10.1016/j.molcel.2017.05.031
Iurlaro R, Munoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283(14):2640–2652. https://doi.org/10.1111/febs.13598
Reinicke AT, Laban K, Sachs M et al (2019) Ubiquitin C-terminal hydrolase L1 (UCH-L1) loss causes neurodegeneration by altering protein turnover in the first postnatal weeks. Proc Natl Acad Sci USA 116(16):7963–7972. https://doi.org/10.1073/pnas.1812413116
Liu S, Gonzalez-Prieto R, Zhang M et al (2020) Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFbeta-induced breast cancer metastasis. Clin Cancer Res 26(6):1460–1473. https://doi.org/10.1158/1078-0432.CCR-19-1373
Costes S, Huang CJ, Gurlo T et al (2011) beta-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes 60(1):227–238. https://doi.org/10.2337/db10-0522
Pippin JW, Brinkkoetter PT, Cormack-Aboud FC et al (2009) Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296(2):F213-229. https://doi.org/10.1152/ajprenal.90421.2008
Garrett MR, Korstanje R (2020) Using genetic and species diversity to tackle kidney disease. Trends Genet 36(7):499–509. https://doi.org/10.1016/j.tig.2020.04.001
Shirato I, Asanuma K, Takeda Y et al (2000) Protein gene product 9.5 is selectively localized in parietal epithelial cells of Bowman’s capsule in the rat kidney. J Am Soc Nephrol 11(12):2381–2386
Ichihara N, Wu J, Chui DH et al (1995) Axonal degeneration promotes abnormal accumulation of amyloid beta-protein in ascending gracile tract of gracile axonal dystrophy (GAD) mouse. Brain Res 695(2):173–178. https://doi.org/10.1016/0006-8993(95)00729-a
Saigoh K, Wang YL, Suh JG et al (1999) Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet 23(1):47–51. https://doi.org/10.1038/12647
Goto A, Wang YL, Kabuta T et al (2009) Proteomic and histochemical analysis of proteins involved in the dying-back-type of axonal degeneration in the gracile axonal dystrophy (gad) mouse. Neurochem Int 54(5–6):330–338. https://doi.org/10.1016/j.neuint.2008.12.012
Fujimoto D, Kuwabara T, Hata Y et al (2020) Suppressed ER-associated degradation by intraglomerular cross talk between mesangial cells and podocytes causes podocyte injury in diabetic kidney disease. FASEB J 34(11):15577–15590. https://doi.org/10.1096/fj.202000078RR
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086. https://doi.org/10.1126/science.1209038
Kopp MC, Larburu N, Durairaj V et al (2019) UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat Struct Mol Biol 26(11):1053–1062. https://doi.org/10.1038/s41594-019-0324-9
Park SJ, Kim Y, Yang SM et al (2019) Discovery of endoplasmic reticulum calcium stabilizers to rescue ER-stressed podocytes in nephrotic syndrome. Proc Natl Acad Sci USA 116(28):14154–14163. https://doi.org/10.1073/pnas.1813580116
Chen YM, Zhou Y, Go G et al (2013) Laminin beta2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J Am Soc Nephrol 24(8):1223–1233. https://doi.org/10.1681/ASN.2012121149
Han J, Back SH, Hur J et al (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15(5):481–490. https://doi.org/10.1038/ncb2738
Chen Y, Gui D, Chen J et al (2014) Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Biochem 33(6):1975–1987. https://doi.org/10.1159/000362974
Yoshida H, Okada T, Haze K et al (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20(18):6755–6767. https://doi.org/10.1128/MCB.20.18.6755-6767.2000
Madhusudhan T, Wang H, Dong W et al (2015) Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun 6:6496. https://doi.org/10.1038/ncomms7496
Shruthi K, Reddy SS, Chitra PS et al (2019) Ubiquitin-proteasome system and ER stress in the brain of diabetic rats. J Cell Biochem 120(4):5962–5973. https://doi.org/10.1002/jcb.27884
Acknowledgements
We would like to thank Dr. Zhihuang Zheng and Chuanlei Li for their help throughout the project.
Funding
This work was supported by the National Natural Science Foundation of China, Grant/Award (no. 82170719).
Author information
Authors and Affiliations
Contributions
HW, ZZ and YX designed the study. YH, CQ, JS and ZZ carried out experiments. WT and AA analyzed data. HW, YH and CQ drafted the paper. HW, YX and ZZ revised the paper. All the authors approved the submitted and published versions of the paper.
Corresponding authors
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The ethics governing the use and conduct of experiments on animals were strictly observed. All animal experiments were approved by the Animal Research Ethics Committee of the School of Basic Medical Sciences of Fudan University (no. 20100223).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Y., Qi, C., Shi, J. et al. Podocyte-specific deletion of ubiquitin carboxyl-terminal hydrolase L1 causes podocyte injury by inducing endoplasmic reticulum stress. Cell. Mol. Life Sci. 80, 106 (2023). https://doi.org/10.1007/s00018-023-04747-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04747-2