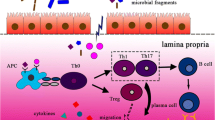
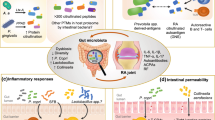
Abstract
Research on the influence of gut microbiota on systemic inflammatory arthritis has exploded in the past decade. Gut microbiota changes may be a crucial regulatory component in systemic inflammatory arthritis. As a result of advancements in the field, microbiota-assisted therapy has evolved, but this discipline is still in its infancy. Consequently, we review the limitations of current systemic inflammatory arthritis treatment, analyze the connection between the microbiota and arthritis, and summarize the research progress of microbiota regulating systemic inflammatory arthritis and the further development aspects of microbiota-assisted therapy. Finally, the partial mechanisms of microbiota-assisted therapy of systemic inflammatory arthritis are being discussed. In general, this review summarizes the current progress, challenges, and prospects of microbiota-assisted therapy for systemic inflammatory arthritis and points out the direction for the development of microbiota-assisted therapy in the future.







Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533. https://doi.org/10.1371/journal.pbio.1002533
Bäckhed F, Ley RE, Sonnenburg JL et al (2005) Host-bacterial mutualism in the human intestine. Science 307:1915. https://doi.org/10.1126/science.1104816
Hildebrand F, Gossmann TI, Frioux C et al (2021) Dispersal strategies shape persistence and evolution of human gut bacteria. Cell Host Microbe 29:1167–1176
Chen L, Wang D, Garmaeva S et al (2021) The long-term genetic stability and individual specificity of the human gut microbiome. Cell 184:2302-2315.e2312. https://doi.org/10.1016/j.cell.2021.03.024
Wu Y, Jiao N, Zhu R et al (2021) Identification of microbial markers across populations in early detection of colorectal cancer. Nat Commun 12:3063. https://doi.org/10.1038/s41467-021-23265-y
Alavi S, Mitchell JD, Cho JY et al (2020) Interpersonal gut microbiome variation drives susceptibility and resistance to cholera infection. Cell 181:1533-1546.e1513. https://doi.org/10.1016/j.cell.2020.05.036
Miyoshi J, Miyoshi S, Delmont TO et al (2021) Early-life microbial restitution reduces colitis risk promoted by antibiotic-induced gut dysbiosis in IL-10-/- mice. Gastroenterology. https://doi.org/10.1053/j.gastro.2021.05.054
Selma-Royo M, Calatayud Arroyo M, García-Mantrana I et al (2020) Perinatal environment shapes microbiota colonization and infant growth: impact on host response and intestinal function. Microbiome 8:167. https://doi.org/10.1186/s40168-020-00940-8
Proctor LM, Creasy HH, Fettweis JM et al (2019) The integrative human microbiome project. Nature 569:641–648. https://doi.org/10.1038/s41586-019-1238-8
Harkins CP, Kong HH, Segre JA (2020) Manipulating the human microbiome to manage disease. JAMA 323:303–304. https://doi.org/10.1001/jama.2019.19602
Clemente JC, Manasson J, Scher JU (2018) The role of the gut microbiome in systemic inflammatory disease. BMJ 360:j5145. https://doi.org/10.1136/bmj.j5145
Rosser EC, Piper CJM, Matei DE et al (2020) Microbiota-derived metabolites suppress arthritis by amplifying Aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab 31:837-851.e810. https://doi.org/10.1016/j.cmet.2020.03.003
Wen C, Zheng Z, Shao T et al (2017) Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol 18:142. https://doi.org/10.1186/s13059-017-1271-6
Gracey E, Vereecke L, McGovern D et al (2020) Revisiting the gut–joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol 16:415–433. https://doi.org/10.1038/s41584-020-0454-9
Fugger L, Jensen LT, Rossjohn J (2020) Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell 181:63–80. https://doi.org/10.1016/j.cell.2020.03.007
Teng F, Klinger CN, Felix KM et al (2016) Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity 44:875–888. https://doi.org/10.1016/j.immuni.2016.03.013
Tracy A, Buckley CD, Raza K (2017) Pre-symptomatic autoimmunity in rheumatoid arthritis: when does the disease start? Sem Immunopathol 39:423–435. https://doi.org/10.1007/s00281-017-0620-6
Zaiss MM, Joyce Wu HJ, Mauro D et al (2021) The gut–joint axis in rheumatoid arthritis. Nat Rev Rheumatol 17:224–237. https://doi.org/10.1038/s41584-021-00585-3
Moentadj R, Wang Y, Bowerman K et al (2021) Streptococcus species enriched in the oral cavity of patients with RA are a source of peptidoglycan-polysaccharide polymers that can induce arthritis in mice. Ann Rheum Dis 80:573. https://doi.org/10.1136/annrheumdis-2020-219009
Behl T, Mehta K, Sehgal A et al (2021) Exploring the role of polyphenols in rheumatoid arthritis. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1924613
Stables MJ, Newson J, Ayoub SS et al (2010) Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and -resistant bacteria. Blood 116:2950–2959. https://doi.org/10.1182/blood-2010-05-284844
Mitchell JA, Warner TD (2006) COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov 5:75–86. https://doi.org/10.1038/nrd1929
Richy F, Bruyere O, Ethgen O et al (2004) Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis 63:759. https://doi.org/10.1136/ard.2003.015925
Bjarnason I, Scarpignato C, Holmgren E et al (2018) Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 154:500–514. https://doi.org/10.1053/j.gastro.2017.10.049
Schjerning A-M, McGettigan P, Gislason G (2020) Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev Cardiol 17:574–584. https://doi.org/10.1038/s41569-020-0366-z
Chan FKL, Lanas A, Scheiman J et al (2010) Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 376:173–179. https://doi.org/10.1016/S0140-6736(10)60673-3
van der Heide A, Jacobs JWG, Bijlsma JWJ et al (1996) The effectiveness of early treatment with “second-line” antirheumatic drugs. Ann Intern Med 124:699–707. https://doi.org/10.7326/0003-4819-124-8-199604150-00001
Macfarlane E, Seibel MJ, Zhou H (2020) Arthritis and the role of endogenous glucocorticoids. Bone Res 8:33. https://doi.org/10.1038/s41413-020-00112-2
George MD, Baker JF, Winthrop K et al (2020) Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis. Ann Intern Med 173:870–878. https://doi.org/10.7326/M20-1594
Burmester GR, Buttgereit F, Bernasconi C et al (2020) Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet 396:267–276. https://doi.org/10.1016/S0140-6736(20)30636-X
Hilliquin S, Hugues B, Mitrovic S et al (2018) Ability of disease-modifying antirheumatic drugs to prevent or delay rheumatoid arthritis onset: a systematic literature review and meta-analysis. Ann Rheum Dis 77:1099. https://doi.org/10.1136/annrheumdis-2017-212612
Verhoeven MMA, de Hair MJH, Tekstra J et al (2019) Initiating tocilizumab, with or without methotrexate, compared with starting methotrexate with prednisone within step-up treatment strategies in early rheumatoid arthritis: an indirect comparison of effectiveness and safety of the U-Act-Early and CAMERA-II treat-to-target trials. Ann Rheum Dis 78:1333. https://doi.org/10.1136/annrheumdis-2019-215304
Dorleijn DMJ, Luijsterburg PAJ, Reijman M et al (2018) Intramuscular glucocorticoid injection versus placebo injection in hip osteoarthritis: a 12-week blinded randomised controlled trial. Ann Rheum Dis 77:875. https://doi.org/10.1136/annrheumdis-2017-212628
Hardy RS, Raza K, Cooper MS (2020) Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat Rev Rheumatol 16:133–144. https://doi.org/10.1038/s41584-020-0371-y
Nabi H, Georgiadis S, Loft AG et al (2021) Comparative effectiveness of two adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator adalimumab: nationwide observational study emulating a randomised clinical trial. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2021-219951
Ikonomidis I, Lekakis JP, Nikolaou M et al (2008) Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 117:2662–2669. https://doi.org/10.1161/CIRCULATIONAHA.107.731877
Pappas DA, St John G, Etzel CJ et al (2021) Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study. Ann Rheum Dis 80:96. https://doi.org/10.1136/annrheumdis-2020-217209
Baraliakos X, Gossec L, Pournara E et al (2021) Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis 80:582. https://doi.org/10.1136/annrheumdis-2020-218808
Armstrong AW, Read C (2020) Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 323:1945–1960. https://doi.org/10.1001/jama.2020.4006
Hresko A, Lin T-C, Solomon DH (2018) Medical care costs associated with rheumatoid arthritis in the US: a systematic literature review and meta-analysis. Arthritis Care Res 70:1431–1438. https://doi.org/10.1002/acr.23512
Kruglov A, Drutskaya M, Schlienz D et al (2020) Contrasting contributions of TNF from distinct cellular sources in arthritis. Ann Rheum Dis 79:1453. https://doi.org/10.1136/annrheumdis-2019-216068
Li Y, Zhang S-X, Yin X-F et al (2021) The gut microbiota and its relevance to peripheral lymphocyte subpopulations and cytokines in patients with rheumatoid arthritis. J Immunol Res. https://doi.org/10.1155/2021/6665563
Yin J, Sternes PR, Wang M et al (2020) Shotgun metagenomics reveals an enrichment of potentially cross-reactive bacterial epitopes in ankylosing spondylitis patients, as well as the effects of TNFi therapy upon microbiome composition. Ann Rheum Dis 79:132. https://doi.org/10.1136/annrheumdis-2019-215763
Shapiro J, Cohen NA, Shalev V et al (2019) Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J Dermatol 46:595–603. https://doi.org/10.1111/1346-8138.14933
Kanaan SB, Sensoy O, Yan Z et al (2019) Immunogenicity of a rheumatoid arthritis protective sequence when acquired through microchimerism. Proc Natl Acad Sci 116:19600. https://doi.org/10.1073/pnas.1904779116
Ye Z, Shen Y, Jin K et al (2021) Arachidonic acid-regulated calcium signaling in T cells from patients with rheumatoid arthritis promotes synovial inflammation. Nat Commun 12:907. https://doi.org/10.1038/s41467-021-21242-z
Terao C, Brynedal B, Chen Z et al (2019) Distinct HLA associations with rheumatoid arthritis subsets defined by serological subphenotype. Am J Hum Genet 105:616–624. https://doi.org/10.1016/j.ajhg.2019.08.002
Van Hoovels L, Jacobs J, Vander Cruyssen B et al (2018) Performance characteristics of rheumatoid factor and anti-cyclic citrullinated peptide antibody assays may impact ACR/EULAR classification of rheumatoid arthritis. Ann Rheum Dis 77:667. https://doi.org/10.1136/annrheumdis-2017-212365
Ishikawa Y, Ikari K, Hashimoto M et al (2019) Shared epitope defines distinct associations of cigarette smoking with levels of anticitrullinated protein antibody and rheumatoid factor. Ann Rheum Dis 78:1480. https://doi.org/10.1136/annrheumdis-2019-215463
Pujades-Rodriguez M, Morgan AW, Cubbon RM et al (2020) Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med 17:e1003432. https://doi.org/10.1371/journal.pmed.1003432
Asquith M, Sternes PR, Costello M-E et al (2019) HLA alleles associated with risk of ankylosing spondylitis and rheumatoid arthritis influence the gut microbiome. Arthr Rheumatol 71:1642–1650. https://doi.org/10.1002/art.40917
Hanberg JS, Hsieh E, Akgün KM et al (2021) Incident rheumatoid arthritis in human immunodeficiency virus infection: epidemiology and treatment. Arthr Rheumatol. https://doi.org/10.1002/art.41802
Kim D, Zeng MY, Núñez G (2017) The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med 49:e339–e339. https://doi.org/10.1038/emm.2017.24
Li D, Feng Y, Tian M et al (2021) Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 9:83. https://doi.org/10.1186/s40168-021-01028-7
Zubeidat K, Hovav A-H (2021) Shaped by the epithelium -postnatal immune mechanisms of oral homeostasis. Trends Immunol 42:622–634. https://doi.org/10.1016/j.it.2021.05.006
Jubair WK, Hendrickson JD, Severs EL et al (2018) Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthr Rheumatol 70:1220–1233. https://doi.org/10.1002/art.40490
Maeda Y, Kurakawa T, Umemoto E et al (2016) Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthr Rheumatol 68:2646–2661. https://doi.org/10.1002/art.39783
Tierney BT, Yang Z, Luber JM et al (2019) The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe 26:283-295.e288. https://doi.org/10.1016/j.chom.2019.07.008
Prodan A, Levin E, Nieuwdorp M (2019) Does disease start in the mouth, the gut or both? Elife 8:e45931. https://doi.org/10.7554/eLife.45931
El-Awady A, de Sousa Rabelo M, Meghil MM et al (2019) Polymicrobial synergy within oral biofilm promotes invasion of dendritic cells and survival of consortia members. npj Biofilms Microbiomes 5:11. https://doi.org/10.1038/s41522-019-0084-7
Okamato Y, Ghosh T, Okamoto T et al (2021) Subjects at-risk for future development of rheumatoid arthritis demonstrate a PAD4-and TLR-dependent enhanced histone H3 citrullination and proinflammatory cytokine production in CD14hi monocytes. J Autoimmun 117:102581. https://doi.org/10.1016/j.jaut.2020.102581
Mondal S, Thompson PR (2019) Protein arginine deiminases (PADs): biochemistry and chemical biology of protein citrullination. Acc Chem Res 52:818–832. https://doi.org/10.1021/acs.accounts.9b00024
Curran AM, Naik P, Giles JT et al (2020) PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets. Nat Rev Rheumatol 16:301–315. https://doi.org/10.1038/s41584-020-0409-1
Montgomery AB, Kopec J, Shrestha L et al (2016) Crystal structure of Porphyromonas gingivalis peptidylarginine deiminase: implications for autoimmunity in rheumatoid arthritis. Ann Rheum Dis 75:1255. https://doi.org/10.1136/annrheumdis-2015-207656
Gabarrini G (2018) Porphyromonas gingivalis, the beast with two heads. A bacterial role in the etiology of rheumatoid arthritis. University of Groningen, Groningen
Lunar Silva I, Cascales E (2021) Molecular strategies underlying Porphyromonas gingivalis virulence. J Mol Biol 433:166836. https://doi.org/10.1016/j.jmb.2021.166836
Takeuchi H, Sasaki N, Yamaga S et al (2019) Porphyromonas gingivalis induces penetration of lipopolysaccharide and peptidoglycan through the gingival epithelium via degradation of junctional adhesion molecule 1. PLoS Pathog 15:e1008124. https://doi.org/10.1371/journal.ppat.1008124
Quirke A-M, Lugli EB, Wegner N et al (2014) Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis 73:263. https://doi.org/10.1136/annrheumdis-2012-202726
Gabarrini G, Grasso S, van Winkelhoff Arie J et al (2020) Gingimaps: protein localization in the oral pathogen Porphyromonas gingivalis. Microbiol Mol Biol Rev 84:e00032-00019. https://doi.org/10.1128/MMBR.00032-19
Glowczyk I, Wong A, Potempa B et al (2017) Inactive gingipains from P. gingivalis selectively skews T cells toward a Th17 phenotype in an IL-6 dependent manner. Front Cell Infect Microbiol 7:140
Maekawa T, Krauss JL, Abe T et al (2014) Porphyromonas gingivalis manipulates complement and TLR Signaling To Uncouple Bacterial Clearance From Inflammation And Promote Dysbiosis. Cell Host Microbe 15:768–778. https://doi.org/10.1016/j.chom.2014.05.012
Manoil D, Bostanci N, Mumcu G et al (2021) Novel and known periodontal pathogens residing in gingival crevicular fluid are associated with rheumatoid arthritis. J Periodontol 92:359–370. https://doi.org/10.1002/JPER.20-0295
Reichert S, Haffner M, Keyßer G et al (2013) Detection of oral bacterial DNA in synovial fluid. J Clin Periodontol 40:591–598. https://doi.org/10.1111/jcpe.12102
Sarwar MT, Ohara-Nemoto Y, Kobayakawa T et al (2020) Characterization of substrate specificity and novel autoprocessing mechanism of dipeptidase A from Prevotella intermedia. Biol Chem 401:629–642. https://doi.org/10.1515/hsz-2019-0387
Tett A, Pasolli E, Masetti G et al (2021) Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. https://doi.org/10.1038/s41579-021-00559-y
Claus SP (2019) The strange case of Prevotella copri: Dr. Jekyll or Mr. Hyde? Cell Host Microbe 26:577–578. https://doi.org/10.1016/j.chom.2019.10.020
Kovatcheva-Datchary P, Nilsson A, Akrami R et al (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22:971–982. https://doi.org/10.1016/j.cmet.2015.10.001
Kishikawa T, Maeda Y, Nii T et al (2020) Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann Rheum Dis 79:103. https://doi.org/10.1136/annrheumdis-2019-215743
Maeda Y, Takeda K (2019) Host–microbiota interactions in rheumatoid arthritis. Exp Mol Med 51:1–6. https://doi.org/10.1038/s12276-019-0283-6
Pianta A, Arvikar SL, Strle K et al (2017) Two rheumatoid arthritis–specific autoantigens correlate microbial immunity with autoimmune responses in joints. J Clin Investig 127:2946–2956. https://doi.org/10.1172/JCI93450
Pisetsky DS (2018) How the gut inflames the joints. Ann Rheum Dis 77:634. https://doi.org/10.1136/annrheumdis-2018-212942
McAllister K, Goodson N, Warburton L et al (2017) Spondyloarthritis: diagnosis and management: summary of NICE guidance. BMJ 356:j839. https://doi.org/10.1136/bmj.j839
Costantino F, Breban M, Garchon H-J (2018) Genetics and functional genomics of spondyloarthritis. Front Immunol 9:2933
Zino E, Terlizzi SD, Carugo C et al (2004) Rapid detection of all HLA-B*27 alleles (B*2701–B*2725) by group-specific polymerase chain reaction. Tissue Antigens 63:88–92. https://doi.org/10.1111/j.1399-0039.2004.00158.x
Parida JR, Kumar S, Ahmed S et al (2021) Reactive arthritis and undifferentiated peripheral spondyloarthritis share human leucocyte antigen B27 subtypes and serum and synovial fluid cytokine profiles. Rheumatology 60:3004–3011. https://doi.org/10.1093/rheumatology/keaa746
Gill T, Asquith M, Brooks SR et al (2018) Effects of HLA–B27 on gut microbiota in experimental spondyloarthritis implicate an ecological model of dysbiosis. Arthr Rheumatol 70:555–565. https://doi.org/10.1002/art.40405
Simone D, Al-Mossawi MH, Bowness P (2018) Progress in our understanding of the pathogenesis of ankylosing spondylitis. Rheumatology 57:vi4–vi9. https://doi.org/10.1093/rheumatology/key001
Khare SD, Luthra HS, David CS (1995) Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking beta 2-microglobulin: a model of human spondyloarthropathies. J Exp Med 182:1153–1158. https://doi.org/10.1084/jem.182.4.1153
Hammer RE, Maika SD, Richardson JA et al (1990) Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell 63:1099–1112. https://doi.org/10.1016/0092-8674(90)90512-D
Qaiyum Z, Gracey E, Yao Y et al (2019) Integrin and transcriptomic profiles identify a distinctive synovial CD8+ T cell subpopulation in spondyloarthritis. Ann Rheum Dis 78:1566. https://doi.org/10.1136/annrheumdis-2019-215349
Guggino G, Rizzo A, Mauro D et al (2019) Gut-derived CD8+ tissue-resident memory T cells are expanded in the peripheral blood and synovia of SpA patients. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2019-216456
Gill T, Rosenbaum JT (2021) Putative pathobionts in HLA-B27-associated spondyloarthropathy. Front Immunol 11:3510
Uchiyama K, Naito Y, Takagi T (2019) Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacol Ther 199:164–172
Voruganti A, Bowness P (2020) New developments in our understanding of ankylosing spondylitis pathogenesis. Immunology 161:94–102. https://doi.org/10.1111/imm.13242
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369:1379–1390. https://doi.org/10.1016/S0140-6736(07)60635-7
Caffrey MFP, James DCO (1973) Human lymphocyte antigen association in ankylosing spondylitis. Nature 242:121–121
Peng Z, Wu J, Wang K et al (2021) Production of a promising biosynthetic self-assembled nanoconjugate vaccine against klebsiella pneumoniae serotype O2 in a general Escherichia coli host. Adv Sci. https://doi.org/10.1002/advs.202100549
Ebringer A, Cowling P, Ngwa-Suh N et al (1976) Cross-reactivity between Klebsiella aerogenes species and B27 lymphocyte antigens as an aetiological factor in ankylosing spondylitis. HLA Dis Paris INSERM 58:27
Seager K, Bashir HV, Geczy AF et al (1979) Evidence for a specific B27-associated cell surface marker on lymphocytes of patients with ankylosing spondylitis. Nature 277:68–70. https://doi.org/10.1038/277068a0
Rashid T, Ebringer A (2007) Ankylosing spondylitis is linked to Klebsiella—the evidence. Clin Rheumatol 26:858–864. https://doi.org/10.1007/s10067-006-0488-7
Stone MA, Payne U, Schentag C et al (2004) Comparative immune responses to candidate arthritogenic bacteria do not confirm a dominant role for Klebsiella pneumonia in the pathogenesis of familial ankylosing spondylitis. Rheumatology 43:148–155. https://doi.org/10.1093/rheumatology/keg482
Liu G, Hao Y, Yang Q et al (2020) The association of fecal microbiota in ankylosing spondylitis cases with C-reactive protein and erythrocyte sedimentation rate. Mediat Inflamm. https://doi.org/10.1155/2020/8884324
Zhou C, Zhao H, Xiao X-Y et al (2020) Metagenomic profiling of the pro-inflammatory gut microbiota in ankylosing spondylitis. J Autoimmun 107:102360
Perez-Chada LM, Haberman RH, Chandran V et al (2021) Consensus terminology for preclinical phases of psoriatic arthritis for use in research studies: results from a Delphi consensus study. Nat Rev Rheumatol 17:238–243. https://doi.org/10.1038/s41584-021-00578-2
Villani AP, Rouzaud M, Sevrain M et al (2015) Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol 73:242–248. https://doi.org/10.1016/j.jaad.2015.05.001
Yegorov S, Babenko D, Kozhakhmetov S et al (2020) Psoriasis is associated with elevated gut IL-1α and intestinal microbiome alterations. Front Immunol 11:2431
Scher JU, Ubeda C, Artacho A et al (2015) Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 67:128–139. https://doi.org/10.1002/art.38892
Arnold JW, Roach J, Fabela S et al (2021) The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 9:1–9
Zhang W, Xu J-H, Yu T et al (2019) Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother 118:109131
Li B, Ge Y, Xu Y et al (2019) Spatial learning and memory impairment in growing mice induced by major oxidized tyrosine product dityrosine. J Agric Food Chem 67:9039–9049. https://doi.org/10.1021/acs.jafc.9b04253
Wang Q, Hernández-Ochoa EO, Viswanathan MC et al (2021) CaMKII oxidation is a critical performance/disease trade-off acquired at the dawn of vertebrate evolution. Nat Commun 12:3175. https://doi.org/10.1038/s41467-021-23549-3
Chakrabarty RP, Chandel NS (2021) Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 28:394–408. https://doi.org/10.1016/j.stem.2021.02.011
Xiong W, MacColl Garfinkel AE, Li Y et al (2015) NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Investig 125:1433–1445. https://doi.org/10.1172/JCI79735
Dumas A, Knaus UG (2021) Raising the ‘good’ oxidants for immune protection. Front Immunol 12:2116
Fan X-X, Xu M-Z, Leung EL-H et al (2020) ROS-responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nano-Micro Letters 12:76. https://doi.org/10.1007/s40820-020-0410-x
Wójcik P, Gęgotek A, Žarković N et al (2021) Oxidative stress and lipid mediators modulate immune cell functions in autoimmune diseases. Int J Mol Sci. https://doi.org/10.3390/ijms22020723
Steinz MM, Santos-Alves E, Lanner JT (2020) Skeletal muscle redox signaling in rheumatoid arthritis. Clin Sci 134:2835–2850. https://doi.org/10.1042/CS20190728
Kristyanto H, Blomberg NJ, Slot LM et al (2020) Persistently activated, proliferative memory autoreactive B cells promote inflammation in rheumatoid arthritis. Sci Transl Med 12:eaaz5327. https://doi.org/10.1126/scitranslmed.aaz5327
Dang PM-C, Stensballe A, Boussetta T et al (2006) A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Investig 116:2033–2043. https://doi.org/10.1172/JCI27544
Wright HL, Lyon M, Chapman EA et al (2021) Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species, and neutrophil extracellular traps. Front Immunol 11:3364
Datta S, Kundu S, Ghosh P et al (2014) Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol 33:1557–1564. https://doi.org/10.1007/s10067-014-2597-z
Kim J, Kim HY, Song SY et al (2019) Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 13:3206–3217. https://doi.org/10.1021/acsnano.8b08785
Su X, Li T, Liu Z et al (2018) Licochalcone A activates Keap1-Nrf2 signaling to suppress arthritis via phosphorylation of p62 at serine 349. Free Radic Biol Med 115:471–483. https://doi.org/10.1016/j.freeradbiomed.2017.12.004
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43:621–626. https://doi.org/10.1042/BST20150014
Zhang W, Feng C, Jiang H (2021) Novel target for treating Alzheimer’s diseases: crosstalk between the Nrf2 pathway and autophagy. Ageing Res Rev 65:101207. https://doi.org/10.1016/j.arr.2020.101207
Gao X, Jiang S, Du Z et al (2019) In vitroKLF2 protects against osteoarthritis by repressing oxidative response through activation of Nrf2/ARE signaling in vitro and in vivo. Oxid Med Cell Longev. https://doi.org/10.1155/2019/8564681
Castejón ML, Alarcón-de-la-Lastra C, Rosillo MÁ et al (2021) A new peracetylated oleuropein derivative ameliorates joint inflammation and destruction in a murine collagen-induced arthritis model via activation of the Nrf-2/Ho-1 antioxidant pathway and suppression of MAPKs and NF-κB activation. Nutrients. https://doi.org/10.3390/nu13020311
Luo Y, Xiong B, Liu H et al (2021) Koumine suppresses IL-1β secretion and attenuates inflammation associated with blocking ROS/NF-κB/NLRP3 axis in macrophages. Front Pharmacol 11:2369
Song H, Zhao C, Yu Z et al (2020) UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun 11:6042. https://doi.org/10.1038/s41467-020-19939-8
Yan Z, Qi W, Zhan J et al (2020) Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med 24:13046–13057. https://doi.org/10.1111/jcmm.15905
Barnes PJ (2020) Oxidative stress-based therapeutics in COPD. Redox Biol 33:101544. https://doi.org/10.1016/j.redox.2020.101544
García S, Bodaño A, Pablos JL et al (2008) Poly(ADP-ribose) polymerase inhibition reduces tumor necrosis factor-induced inflammatory response in rheumatoid synovial fibroblasts. Ann Rheum Dis 67:631. https://doi.org/10.1136/ard.2007.077040
Chadha S, Behl T, Kumar A et al (2020) Role of Nrf2 in rheumatoid arthritis. Curr Res Transl Med 68:171–181. https://doi.org/10.1016/j.retram.2020.05.002
Na H-K, Surh Y-J (2006) Transcriptional regulation via cysteine thiol modification: a novel molecular strategy for chemoprevention and cytoprotection. Mol Carcinog 45:368–380. https://doi.org/10.1002/mc.20225
Tang K-T, Lin C-C, Lin S-C et al (2021) Kurarinone attenuates collagen-induced arthritis in mice by inhibiting Th1/Th17 cell responses and oxidative stress. Int J Mol Sci. https://doi.org/10.3390/ijms22084002
Sánchez Macarro M, Ávila-Gandía V, Pérez-Piñero S et al (2021) Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants. https://doi.org/10.3390/antiox10020323
Hao L, Cheng Y, Su W et al (2021) Pediococcus pentosaceus ZJUAF-4 relieves oxidative stress and restores the gut microbiota in diquat-induced intestinal injury. Appl Microbiol Biotechnol 105:1657–1668. https://doi.org/10.1007/s00253-021-11111-6
Monteros MJM, Galdeano CM, Balcells MF et al (2021) Probiotic lactobacilli as a promising strategy to ameliorate disorders associated with intestinal inflammation induced by a non-steroidal anti-inflammatory drug. Sci Rep 11:571. https://doi.org/10.1038/s41598-020-80482-z
Richards JL, Yap YA, McLeod KH et al (2016) Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunol 5:e82. https://doi.org/10.1038/cti.2016.29
Topping DL, Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064. https://doi.org/10.1152/physrev.2001.81.3.1031
Qiao S, Lian X, Yue M et al (2020) Regulation of gut microbiota substantially contributes to the induction of intestinal Treg cells and consequent anti-arthritis effect of madecassoside. Int Immunopharmacol 89:107047. https://doi.org/10.1016/j.intimp.2020.107047
Mehta H, Mashiko S, Angsana J et al (2021) Differential changes in inflammatory mononuclear phagocyte and T-cell profiles within psoriatic skin during treatment with Guselkumab vs Secukinumab. J Investig Dermatol 141:1707-1718.e1709. https://doi.org/10.1016/j.jid.2021.01.005
Shen B, Hu J, Song H et al (2019) Antibiotics exacerbated colitis by affecting the microbiota, Treg cells and SCFAs in IL10-deficient mice. Biomed Pharmacother 114:108849. https://doi.org/10.1016/j.biopha.2019.108849
Bai Y, Li Y, Marion T et al (2021) Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J Autoimmun 116:102564. https://doi.org/10.1016/j.jaut.2020.102564
Cao T, Zhang X, Chen D et al (2018) The epigenetic modification during the induction of Foxp3 with sodium butyrate. Immunopharmacol Immunotoxicol 40:309–318. https://doi.org/10.1080/08923973.2018.1480631
Fan Z, Yang B, Ross RP et al (2020) Protective effects of Bifidobacterium adolescentis on collagen-induced arthritis in rats depend on timing of administration. Food Funct 11:4499–4511. https://doi.org/10.1039/D0FO00077A
Hui W, Yu D, Cao Z et al (2019) Butyrate inhibit collagen-induced arthritis via Treg/IL-10/Th17 axis. Int Immunopharmacol 68:226–233. https://doi.org/10.1016/j.intimp.2019.01.018
Balmer ML, Ma EH, Bantug GR et al (2016) Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 44:1312–1324. https://doi.org/10.1016/j.immuni.2016.03.016
Park J, Kim M, Kang SG et al (2015) Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol 8:80–93. https://doi.org/10.1038/mi.2014.44
Hernandez-Sanabria E, Heiremans E, Calatayud Arroyo M et al (2020) Short-term supplementation of celecoxib-shifted butyrate production on a simulated model of the gut microbial ecosystem and ameliorated in vitro inflammation. npj Biofilms Microbiomes 6:9. https://doi.org/10.1038/s41522-020-0119-0
Verschueren KHG, Blanchet C, Felix J et al (2019) Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature 568:571–575. https://doi.org/10.1038/s41586-019-1095-5
Lauterbach MA, Hanke JE, Serefidou M et al (2019) Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity 51:997-1011.e1017. https://doi.org/10.1016/j.immuni.2019.11.009
Luu M, Visekruna A (2019) Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol 49:842–848. https://doi.org/10.1002/eji.201848009
Wiechers C, Zou M, Galvez E et al (2021) The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell Mol Immunol 18:1211–1221. https://doi.org/10.1038/s41423-021-00647-2
Atanes P, Ashik T, Persaud SJ (2021) Obesity-induced changes in human islet G protein-coupled receptor expression: Implications for metabolic regulation. Pharmacol Ther. https://doi.org/10.1016/j.pharmthera.2021.107928
Brown AJ, Goldsworthy SM, Barnes AA et al (2003) The orphan G Protein-Coupled Receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids *. J Biol Chem 278:11312–11319. https://doi.org/10.1074/jbc.M211609200
Fu S-P, Wang J-F, Xue W-J et al (2015) Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflamm 12:9. https://doi.org/10.1186/s12974-014-0230-3
Kim MH, Kang SG, Park JH et al (2013) Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145:396–406
Smith PM, Howitt MR, Panikov N et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341:569–573
Maslowski KM, Vieira AT, Ng A et al (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286. https://doi.org/10.1038/nature08530
Flak MB, Colas RA, Muñoz-Atienza E et al (2019) Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight. https://doi.org/10.1172/jci.insight.125191
Fakhoury HMA, Kvietys PR, AlKattan W et al (2020) Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol 200:105663. https://doi.org/10.1016/j.jsbmb.2020.105663
Brandl C, Bucci L, Schett G et al (2021) Crossing the barriers: revisiting the gut feeling in rheumatoid arthritis. Eur J Immunol 51:798–810. https://doi.org/10.1002/eji.202048876
Pedersen SJ, Maksymowych WP (2019) The pathogenesis of ankylosing spondylitis: an update. Curr Rheumatol Rep 21:58. https://doi.org/10.1007/s11926-019-0856-3
Groschwitz KR, Hogan SP (2009) Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124:3–22. https://doi.org/10.1016/j.jaci.2009.05.038
Krause G, Winkler L, Mueller SL et al (2008) Structure and function of claudins. Biochim Biophys Acta (BBA) 1778:631–645. https://doi.org/10.1016/j.bbamem.2007.10.018
Turksen K, Troy T-C (2004) Barriers built on claudins. J Cell Sci 117:2435–2447. https://doi.org/10.1242/jcs.01235
Chánez-Paredes S, Montoya-García A, Castro-Ochoa KF et al (2021) The Arp2/3 inhibitory protein arpin is required for intestinal epithelial barrier integrity. Front Cell Dev Biol 9:829
Shi H, Yu Y, Lin D et al (2020) β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 8:143. https://doi.org/10.1186/s40168-020-00920-y
Gomez A, Luckey D, Yeoman CJ et al (2012) Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE 7:e36095
Fasano A (2011) Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 91:151–175
Tajik N, Frech M, Schulz O et al (2020) Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun 11:1995. https://doi.org/10.1038/s41467-020-15831-7
Ciccia F, Guggino G, Rizzo A et al (2017) Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis 76:1123. https://doi.org/10.1136/annrheumdis-2016-210000
Rizzo A, Ferrante A, Guggino G et al (2017) Gut inflammation in spondyloarthritis. Best Pract Res Clin Rheumatol 31:863–876. https://doi.org/10.1016/j.berh.2018.08.012
Chen Y, Yang B, Stanton C et al (2021) Bifidobacterium pseudocatenulatum ameliorates DSS-induced colitis by maintaining intestinal mechanical barrier, blocking proinflammatory cytokines, inhibiting TLR4/NF-κB signaling, and altering gut microbiota. J Agric Food Chem 69:1496–1512. https://doi.org/10.1021/acs.jafc.0c06329
Gao Y, Liu Y, Ma F et al (2021) Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food Funct 12:373–386. https://doi.org/10.1039/D0FO02794D
Hu T, Wang H, Xiang C et al (2020) Lactobacillus acidophilus preventive effect of XY27 on DSS-induced ulcerative colitis in mice. Drug Des Devel Ther 14:5645–5657. https://doi.org/10.2147/DDDT.S284422
Jia H, Ren S, Wang X (2019) Heat-killed probiotic regulates the body’s regulatory immunity to attenuate subsequent experimental autoimmune arthritis. Immunol Lett 216:89–96. https://doi.org/10.1016/j.imlet.2019.10.009
Jhun J, Min H-K, Na HS et al (2020) Combinatmarion treatment with Lactobacillus acidophilus LA-1, vitamin B, and curcumin ameliorates the progression of osteoarthritis by inhibiting the pro-inflammatory mediators. Immunol Lett 228:112–121. https://doi.org/10.1016/j.imlet.2020.10.008
Yamashita M, Matsumoto K, Endo T et al (2017) Preventive effect of Lactobacillus helveticus SBT2171 on collagen-induced arthritis in mice. Front Microbiol 8:1159
Salminen S, Collado MC, Endo A et al (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-021-00440-6
Nowak B, Śróttek M, Ciszek-Lenda M et al (2020) Exopolysaccharide from Lactobacillus rhamnosus KL37 Inhibits T Cell-dependent Immune Response in Mice. Arch Immunol Ther Exp 68:17. https://doi.org/10.1007/s00005-020-00581-7
Liu Y, Alookaran JJ, Rhoads JM (2018) Probiotics in autoimmune and inflammatory disorders. Nutrients. https://doi.org/10.3390/nu10101537
Liu Y, Aryee MJ, Padyukov L et al (2013) Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat Biotechnol 31:142–147. https://doi.org/10.1038/nbt.2487
Huang Y, Wang H, Ba X et al (2020) Decipher manifestations and Treg /Th17 imbalance in multi-staging rheumatoid arthritis and correlation with TSDR/RORC methylation. Mol Immunol 127:1–11. https://doi.org/10.1016/j.molimm.2020.08.002
Ai R, Boyle DL, Wang W et al (2021) Distinct DNA methylation patterns of rheumatoid arthritis peripheral blood and synovial tissue T cells. ACR Open Rheumatol 3:127–132. https://doi.org/10.1002/acr2.11231
Ghadimi D, Helwig U, Schrezenmeir J et al (2012) Epigenetic imprinting by commensal probiotics inhibits the IL-23/IL-17 axis in an in vitro model of the intestinal mucosal immune system. J Leukoc Biol 92:895–911. https://doi.org/10.1189/jlb.0611286
Takahashi K, Sugi Y, Nakano K et al (2011) Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem 286:35755–35762
Shulman Z, Stern-Ginossar N (2020) The RNA modification N6-methyladenosine as a novel regulator of the immune system. Nat Immunol 21:501–512. https://doi.org/10.1038/s41590-020-0650-4
Luo Q, Gao Y, Zhang L et al (2020) Decreased ALKBH5, FTO, and YTHDF2 in peripheral blood are as risk factors for rheumatoid arthritis. Biomed Res Int. https://doi.org/10.1155/2020/5735279
Jiang H, Cao K, Fan C et al (2021) Transcriptome-wide high-throughput m6A sequencing of differential m6A methylation patterns in the human rheumatoid arthritis fibroblast-like synoviocytes cell line MH7A. J Inflamm Res 14:575
Jabs S, Biton A, Bécavin C et al (2020) Impact of the gut microbiota on the m6A epitranscriptome of mouse cecum and liver. Nat Commun 11:1344. https://doi.org/10.1038/s41467-020-15126-x
Suzuki A, Guerrini MM, Yamamoto K (2021) Functional genomics of autoimmune diseases. Ann Rheum Dis 80:689. https://doi.org/10.1136/annrheumdis-2019-216794
Cunningham CC, Wade S, Floudas A et al (2021) Serum miRNA signature in rheumatoid arthritis and “at-risk individuals.” Front Immunol 12:126
Lin S-H, Ho J-C, Li S-C et al (2020) Upregulation of miR-941 in circulating CD14+ monocytes enhances osteoclast activation via WNT16 inhibition in patients with psoriatic arthritis. Int J Mol Sci. https://doi.org/10.3390/ijms21124301
Chen L, Al-Mossawi MH, Ridley A et al (2017) miR-10b-5p is a novel Th17 regulator present in Th17 cells from ankylosing spondylitis. Ann Rheum Dis 76:620. https://doi.org/10.1136/annrheumdis-2016-210175
Rao Y, Fang Y, Tan W et al (2020) Delivery of long non-coding RNA NEAT1 by peripheral blood monouclear cells-derived exosomes promotes the occurrence of rheumatoid arthritis via the MicroRNA-23a/MDM2/SIRT6 Axis. Front Cell Dev Biol 8:952
Tang X, Wang J, Xia X et al (2019) Elevated expression of ciRS-7 in peripheral blood mononuclear cells from rheumatoid arthritis patients. Diagn Pathol 14:11. https://doi.org/10.1186/s13000-019-0783-7
Rodríguez-Nogales A, Algieri F, Garrido-Mesa J et al (2017) Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: impact on microRNAs expression and microbiota composition. Mol Nutr Food Res 61:1700144. https://doi.org/10.1002/mnfr.201700144
Hou Q, Huang Y, Wang Y et al (2020) Lactobacillus casei LC01 regulates intestinal epithelial permeability through miR-144 targeting of OCLN and ZO1. J Microbiol Biotechnol 30:1480–1487. https://doi.org/10.4014/jmb.2002.02059
Thomson DW, Dinger ME (2016) Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17:272–283. https://doi.org/10.1038/nrg.2016.20
Tian P, O’Riordan KJ, Lee Y-K et al (2020) Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress 12:100216. https://doi.org/10.1016/j.ynstr.2020.100216
Bhanja S, Mohanakumar KP (2010) Early-life treatment of antiserotonin antibodies alters sensitivity to serotonin receptors, nociceptive stimulus and serotonin metabolism in adult rats. Int J Dev Neurosci 28:317–324. https://doi.org/10.1016/j.ijdevneu.2010.02.007
Yeoh N, Burton JP, Suppiah P et al (2013) The role of the microbiome in rheumatic diseases. Curr Rheumatol Rep 15:314. https://doi.org/10.1007/s11926-012-0314-y
Hanssen NMJ, de Vos WM, Nieuwdorp M (2021) Fecal microbiota transplantation in human metabolic diseases: from a murky past to a bright future? Cell Metab 33:1098–1110. https://doi.org/10.1016/j.cmet.2021.05.005
Danne C, Rolhion N, Sokol H (2021) Recipient factors in faecal microbiota transplantation: one stool does not fit all. Nat Rev Gastroenterol Hepatol 18:503–513. https://doi.org/10.1038/s41575-021-00441-5
Zeng J, Peng L, Zheng W et al (2021) Fecal microbiota transplantation for rheumatoid arthritis: a case report. Clin Case Rep 9:906–909. https://doi.org/10.1002/ccr3.3677
Kragsnaes MS, Kjeldsen J, Horn HC et al (2021) Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: an exploratory randomised placebo-controlled trial. Ann Rheum Dis 80:1158. https://doi.org/10.1136/annrheumdis-2020-219511
Funding
This research was supported by the National Natural Science Foundation of China (Nos. 32021005, 31820103010), and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Author information
Authors and Affiliations
Contributions
WC and BY supervised the study. RPR, CS and HZ participated in the design and discussion of the research. BL, BY, XL, JZ, RPR, CS, HZ, and WC contributed to the analysis, and interpretation for the work. BL drafted manuscript. BY, RPR and WC revised the manuscript and all authors have read and approved final interpretation for the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
The manuscript does not contain human research, and any individual person’s data in any form (including any individual details, images or videos).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, B., Yang, B., Liu, X. et al. Microbiota-assisted therapy for systemic inflammatory arthritis: advances and mechanistic insights. Cell. Mol. Life Sci. 79, 470 (2022). https://doi.org/10.1007/s00018-022-04498-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04498-6