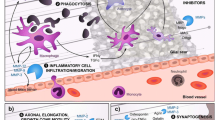
Abstract
Neurotrauma, a term referencing both traumatic brain and spinal cord injuries, is unique to neurodegeneration in that onset is clearly defined. From the perspective of matrix metalloproteinases (MMPs), there is opportunity to define their temporal participation in injury and recovery beginning at the level of the synapse. Here we examine the diverse roles of MMPs in the context of targeted insults (optic nerve lesion and hippocampal and olfactory bulb deafferentation), and clinically relevant focal models of traumatic brain and spinal cord injuries. Time-specific MMP postinjury signaling is critical to synaptic recovery after focal axonal injuries; members of the MMP family exhibit a signature temporal profile corresponding to axonal degeneration and regrowth, where they direct postinjury reorganization and synaptic stabilization. In both traumatic brain and spinal cord injuries, MMPs mediate early secondary pathogenesis including disruption of the blood–brain barrier, creating an environment that may be hostile to recovery. They are also critical players in wound healing including angiogenesis and the formation of an inhibitory glial scar. Experimental strategies to reduce their activity in the acute phase result in long-term neurological recovery after neurotrauma and have led to the first clinical trial in spinal cord injured pet dogs.





Similar content being viewed by others
References
Phillips LL, Chan JL, Doperalski AE, Reeves TM (2014) Time dependent integration of matrix metalloproteinases and their targeted substrates directs axonal sprouting and synaptogenesis following central nervous system injury. Neural Regener Res 9(4):362–376. https://doi.org/10.4103/1673-5374.128237
Andries L, Van Hove I, Moons L, De Groef L (2017) Matrix metalloproteinases during axonal regeneration, a multifactorial role from start to finish. Mol Neurobiol 54(3):2114–2125. https://doi.org/10.1007/s12035-016-9801-x
Fischer D, Harvey AR, Pernet V, Lemmon VP, Park KK (2017) Optic nerve regeneration in mammals: regenerated or spared axons? Exp Neurol 296:83–88. https://doi.org/10.1016/j.expneurol.2017.07.008
Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A (2005) Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci 28(1):64–78. https://doi.org/10.1016/j.mcn.2004.08.013
Diekmann H, Kalbhen P, Fischer D (2015) Characterization of optic nerve regeneration using transgenic zebrafish. Front Cell Neurosci 9:118. https://doi.org/10.3389/fncel.2015.00118
Liu LY, Zheng H, Xiao HL, She ZJ, Zhao SM, Chen ZL, Zhou GM (2008) Comparison of blood-nerve barrier disruption and matrix metalloprotease-9 expression in injured central and peripheral nerves in mice. Neurosci Lett 434(2):155–159. https://doi.org/10.1016/j.neulet.2007.12.052
Hughes PM, Wells GM, Perry VH, Brown MC, Miller KM (2002) Comparison of matrix metalloproteinase expression during Wallerian degeneration in the central and peripheral nervous systems. Neuroscience 113(2):273–287
Lemmens K, Bollaerts I, Bhumika S, de Groef L, Van Houcke J, Darras VM, Van Hove I, Moons L (2016) Matrix metalloproteinases as promising regulators of axonal regrowth in the injured adult zebrafish retinotectal system. J Comp Neurol 524(7):1472–1493. https://doi.org/10.1002/cne.23920
Kim HJ, Fillmore HL, Reeves TM, Phillips LL (2005) Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol 192(1):60–72. https://doi.org/10.1016/j.expneurol.2004.10.014
Mayer J, Hamel MG, Gottschall PE (2005) Evidence for proteolytic cleavage of brevican by the ADAMTSs in the dentate gyrus after excitotoxic lesion of the mouse entorhinal cortex. BMC Neurosci 6:52. https://doi.org/10.1186/1471-2202-6-52
Falo MC, Fillmore HL, Reeves TM, Phillips LL (2006) Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res 84(4):768–781. https://doi.org/10.1002/jnr.20986
Chan JL, Reeves TM, Phillips LL (2014) Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp Neurol 261:757–771. https://doi.org/10.1016/j.expneurol.2014.08.015
Warren KM, Reeves TM, Phillips LL (2012) MT5-MMP, ADAM-10, and N-cadherin act in concert to facilitate synapse reorganization after traumatic brain injury. J Neurotrauma 29(10):1922–1940. https://doi.org/10.1089/neu.2012.2383
Costanzo RM, Perrino LA (2008) Peak in matrix metalloproteinases-2 levels observed during recovery from olfactory nerve injury. NeuroReport 19(3):327–331. https://doi.org/10.1097/WNR.0b013e3282f50c7b
Costanzo RM, Perrino LA, Kobayashi M (2006) Response of matrix metalloproteinase-9 to olfactory nerve injury. NeuroReport 17(17):1787–1791. https://doi.org/10.1097/WNR.0b013e32800fef87
Bakos SR, Costanzo RM (2011) Matrix metalloproteinase-9 is associated with acute inflammation after olfactory injury. NeuroReport 22(11):539–543. https://doi.org/10.1097/WNR.0b013e328348ab94
Bakos SR, Schwob JE, Costanzo RM (2010) Matrix metalloproteinase-9 and -2 expression in the olfactory bulb following methyl bromide gas exposure. Chem Senses 35(8):655–661. https://doi.org/10.1093/chemse/bjq056
Morrison EE, Costanzo RM (1995) Regeneration of olfactory sensory neurons and reconnection in the aging hamster central nervous system. Neurosci Lett 198(3):213–217
Ladwig A, Walter HL, Hucklenbroich J, Willuweit A, Langen KJ, Fink GR, Rueger MA, Schroeter M (2017) Osteopontin augments M2 microglia response and separates M1- and M2-polarized microglial activation in permanent focal cerebral ischemia. Mediat Inflamm 2017:7189421. https://doi.org/10.1155/2017/7189421
Kong M, Munoz N, Valdivia A, Alvarez A, Herrera-Molina R, Cardenas A, Schneider P, Burridge K, Quest AF (1833) Leyton L (2013) Thy-1-mediated cell-cell contact induces astrocyte migration through the engagement of alphaVbeta3 integrin and syndecan-4. Biochem Biophys Acta 6:1409–1420. https://doi.org/10.1016/j.bbamcr.2013.02.013
Peluffo H, Gonzalez P, Aris A, Acarin L, Saura J, Villaverde A, Castellano B, Gonzalez B (2007) RGD domains neuroprotect the immature brain by a glial-dependent mechanism. Ann Neurol 62(3):251–261. https://doi.org/10.1002/ana.21170
Lindsey ML, Zouein FA, Tian Y, Padmanabhan Iyer R, de Castro Bras LE (2015) Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can J Physiol Pharmacol 93(10):879–886. https://doi.org/10.1139/cjpp-2015-0019
Scatena M, Liaw L, Giachelli CM (2007) Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 27(11):2302–2309. https://doi.org/10.1161/ATVBAHA.107.144824
Gaublomme D, Buyens T, De Groef L, Stakenborg M, Janssens E, Ingvarsen S, Porse A, Behrendt N, Moons L (2014) Matrix metalloproteinase 2 and membrane type 1 matrix metalloproteinase co-regulate axonal outgrowth of mouse retinal ganglion cells. J Neurochem 129(6):966–979. https://doi.org/10.1111/jnc.12703
Lagos-Cabre R, Alvarez A, Kong M, Burgos-Bravo F, Cardenas A, Rojas-Mancilla E, Perez-Nunez R, Herrera-Molina R, Rojas F, Schneider P, Herrera-Marschitz M, Quest AFG, van Zundert B, Leyton L (2017) alphaVbeta3 Integrin regulates astrocyte reactivity. J Neuroinflamm 14(1):194. https://doi.org/10.1186/s12974-017-0968-5
Falo MC, Reeves TM, Phillips LL (2008) Agrin expression during synaptogenesis induced by traumatic brain injury. J Neurotrauma 25(7):769–783. https://doi.org/10.1089/neu.2008.0511
Harris JL, Reeves TM, Phillips LL (2009) Injury modality, survival interval, and sample region are critical determinants of qRT-PCR reference gene selection during long-term recovery from brain trauma. J Neurotrauma 26(10):1669–1681. https://doi.org/10.1089/neu.2009-0875
Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH (2002) Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res 100(1–2):103–117
Ng MT, Stammers AT, Kwon BK (2011) Vascular disruption and the role of angiogenic proteins after spinal cord injury. Transl Stroke Res 2(4):474–491. https://doi.org/10.1007/s12975-011-0109-x
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) Blood–brain barrier: from physiology to disease and back. Physiol Rev 99(1):21–78. https://doi.org/10.1152/physrev.00050.2017
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology 7(4):452–464. https://doi.org/10.1215/S1152851705000232
Maikos JT, Shreiber DI (2007) Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma 24(3):492–507. https://doi.org/10.1089/neu.2006.0149
Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ (2003) Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res 74(2):227–239. https://doi.org/10.1002/jnr.10759
Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, Narayana PA (2009) Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed 22(3):332–341. https://doi.org/10.1002/nbm.1343
Popovich PG, Horner PJ, Mullin BB, Stokes BT (1996) A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp Neurol 142(2):258–275. https://doi.org/10.1006/exnr.1996.0196
Tanno H, Nockels RP, Pitts LH, Noble LJ (1992) Breakdown of the blood–brain barrier after fluid percussive brain injury in the rat. Part 1: Distribution and time course of protein extravasation. J Neurotrauma 9(1):21–32. https://doi.org/10.1089/neu.1992.9.21
Dhillon HS, Donaldson D, Dempsey RJ, Prasad MR (1994) Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J Neurotrauma 11(4):405–415. https://doi.org/10.1089/neu.1994.11.405
Baskaya MK, Rao AM, Dogan A, Donaldson D, Dempsey RJ (1997) The biphasic opening of the blood–brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett 226(1):33–36
Cortez SC, McIntosh TK, Noble LJ (1989) Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res 482(2):271–282
Fukuda K, Tanno H, Okimura Y, Nakamura M, Yamaura A (1995) The blood–brain barrier disruption to circulating proteins in the early period after fluid percussion brain injury in rats. J Neurotrauma 12(3):315–324. https://doi.org/10.1089/neu.1995.12.315
Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR (2007) Changes in blood–brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci 25(1):231–238. https://doi.org/10.1111/j.1460-9568.2006.05275.x
Chodobski A, Zink BJ, Szmydynger-Chodobska J (2011) Blood–brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2(4):492–516. https://doi.org/10.1007/s12975-011-0125-x
Saunders NR, Dziegielewska KM, Mollgard K, Habgood MD (2015) Markers for blood–brain barrier integrity: how appropriate is Evans blue in the twenty-first century and what are the alternatives? Front Neurosci 9:385. https://doi.org/10.3389/fnins.2015.00385
Prakash R, Carmichael ST (2015) Blood–brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol 28(6):556–564. https://doi.org/10.1097/WCO.0000000000000248
Glushakova OY, Johnson D, Hayes RL (2014) Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood–brain barrier disruption, and progressive white matter damage. J Neurotrauma 31(13):1180–1193. https://doi.org/10.1089/neu.2013.3080
Cordonnier C, van der Flier WM (2011) Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain J Neurol 134(Pt 2):335–344. https://doi.org/10.1093/brain/awq321
McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68(7):709–735. https://doi.org/10.1097/NEN.0b013e3181a9d503
Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH (2000) Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cerebral Blood Flow Metabol 20(12):1681–1689. https://doi.org/10.1097/00004647-200012000-00007
Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z (2002) Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci 22(17):7526–7535
Trivedi A, Lee SM, Zhang H, Linda J, Noble-Haeusslein L (2014) Neutrophils as determinants of vascular stability in the injured spinal cord. In: Eng H, Lo JL, MingMing N, Michael JW (eds) Vascular mechanisms in CNS trauma. Springer Series in Translational Stroke Research. Springer, New York, pp 285–302
Zhang H, Adwanikar H, Werb Z, Noble-Haeusslein LJ (2010) Matrix metalloproteinases and neurotrauma: evolving roles in injury and reparative processes. Neurosci 16(2):156–170. https://doi.org/10.1177/1073858409355830
Abdul-Muneer PM, Pfister BJ, Haorah J, Chandra N (2016) Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Mol Neurobiol 53(9):6106–6123. https://doi.org/10.1007/s12035-015-9520-8
Jha RM, Kochanek PM, Simard JM (2018) Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2018.08.004
Kumar H, Ropper AE, Lee SH, Han I (2017) Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol 54(5):3578–3590. https://doi.org/10.1007/s12035-016-9910-6
Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L (2012) Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. J Neurochem 123(2):203–216. https://doi.org/10.1111/j.1471-4159.2012.07900.x
Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH (2007) Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery 61(5):1067–1075. https://doi.org/10.1227/01.neu.0000303203.07866.18 (discussion 1075–1066)
Yang Y, Rosenberg GA (2015) Matrix metalloproteinases as therapeutic targets for stroke. Brain Res 1623:30–38. https://doi.org/10.1016/j.brainres.2015.04.024
Sulhan S, Lyon KA, Shapiro LA, Huang JH (2018) Neuroinflammation and blood–brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J Neurosci Res. https://doi.org/10.1002/jnr.24331
Kumar H, Jo MJ, Choi H, Muttigi MS, Shon S, Kim BJ, Lee SH, Han IB (2018) Matrix metalloproteinase-8 inhibition prevents disruption of blood-spinal cord barrier and attenuates inflammation in rat model of spinal cord injury. Mol Neurobiol 55(3):2577–2590. https://doi.org/10.1007/s12035-017-0509-3
Lee JY, Choi HY, Ahn HJ, Ju BG, Yune TY (2014) Matrix metalloproteinase-3 promotes early blood-spinal cord barrier disruption and hemorrhage and impairs long-term neurological recovery after spinal cord injury. Am J Pathol 184(11):2985–3000. https://doi.org/10.1016/j.ajpath.2014.07.016
Chelluboina B, Nalamolu KR, Klopfenstein JD, Pinson DM, Wang DZ, Vemuganti R, Veeravalli KK (2018) MMP-12, a promising therapeutic target for neurological diseases. Mol Neurobiol 55(2):1405–1409. https://doi.org/10.1007/s12035-017-0418-5
Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW (2003) An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci 23(31):10107–10115
Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM, Werb Z, Noble-Haeusslein LJ (2011) Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. J Neurosci 31(44):15894–15903. https://doi.org/10.1523/JNEUROSCI.3943-11.2011
Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L, White S, Basso DM (2013) Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci 33(32):13101–13111. https://doi.org/10.1523/JNEUROSCI.1576-13.2013
Hansen CN, Norden DM, Faw TD, Deibert R, Wohleb ES, Sheridan JF, Godbout JP, Basso DM (2016) Lumbar myeloid cell trafficking into locomotor networks after thoracic spinal cord injury. Exp Neurol 282:86–98. https://doi.org/10.1016/j.expneurol.2016.05.019
Simard JM, Woo SK, Aarabi B, Gerzanich V (2013) The Sur1-Trpm4 channel in spinal cord injury. J Spine. https://doi.org/10.4172/2165-7939.s4-002
Mahoney ET, Benton RL, Maddie MA, Whittemore SR, Hagg T (2009) ADAM8 is selectively up-regulated in endothelial cells and is associated with angiogenesis after spinal cord injury in adult mice. J Comp Neurol 512(2):243–255. https://doi.org/10.1002/cne.21902
Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, Cun CL, Werb Z, Noble-Haeusslein LJ (2008) Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci 28(50):13467–13477. https://doi.org/10.1523/JNEUROSCI.2287-08.2008
Trivedi A, Zhang H, Ekeledo A, Lee S, Werb Z, Plant GW, Noble-Haeusslein LJ (2016) Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord. Exp Neurol 284(Pt A):50–62. https://doi.org/10.1016/j.expneurol.2016.07.018
Zhang Y, Xiong Y, Mahmood A, Zhang ZG, Chopp M (2014) Angiogenesis and functional recovery after traumatic brain injury. In: Eng H, Lo JL, MingMing N, Michael JW (eds) Vascular mechanisms. Springer Series in Translational Stroke Research. Springer, New York, pp 141–156
Renault-Mihara F, Mukaino M, Shinozaki M, Kumamaru H, Kawase S, Baudoux M, Ishibashi T, Kawabata S, Nishiyama Y, Sugai K, Yasutake K, Okada S, Nakamura M, Okano H (2017) Regulation of RhoA by STAT3 coordinates glial scar formation. J Cell Biol 216(8):2533–2550. https://doi.org/10.1083/jcb.201610102
Verslegers M, Lemmens K, Van Hove I, Moons L (2013) Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol 105:60–78. https://doi.org/10.1016/j.pneurobio.2013.03.004
Chetty C, Lakka SS, Bhoopathi P, Rao JS (2010) MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer 127(5):1081–1095. https://doi.org/10.1002/ijc.25134
Han S, Arnold SA, Sithu SD, Mahoney ET, Geralds JT, Tran P, Benton RL, Maddie MA, D’Souza SE, Whittemore SR, Hagg T (2010) Rescuing vasculature with intravenous angiopoietin-1 and alpha v beta 3 integrin peptide is protective after spinal cord injury. Brain J Neurol 133(Pt 4):1026–1042. https://doi.org/10.1093/brain/awq034
Mundel TM, Kalluri R (2007) Type IV collagen-derived angiogenesis inhibitors. Microvasc Res 74(2–3):85–89. https://doi.org/10.1016/j.mvr.2007.05.005
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468(7323):562–566. https://doi.org/10.1038/nature09513
Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV (2012) Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cerebral Blood Flow Metabol 32(10):1841–1852. https://doi.org/10.1038/jcbfm.2012.113
Geevarghese A, Herman IM (2014) Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 163(4):296–306. https://doi.org/10.1016/j.trsl.2014.01.011
Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG (2017) Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun 8:16106. https://doi.org/10.1038/ncomms16106
Risinger GM Jr, Updike DL, Bullen EC, Tomasek JJ, Howard EW (2010) TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol 298(1):C191–C201. https://doi.org/10.1152/ajpcell.00417.2008
Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM (2012) Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120(7):1516–1527. https://doi.org/10.1182/blood-2011-01-332338
Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81(2):229–248. https://doi.org/10.1016/j.neuron.2013.12.034
Tran AP, Warren PM, Silver J (2018) The biology of regeneration failure and success after spinal cord injury. Physiol Rev 98(2):881–917. https://doi.org/10.1152/physrev.00017.2017
Zhou Y, Cui Z, Xia X, Liu C, Zhu X, Cao J, Wu Y, Zhou L, Ben Z, Song Y, Zhang H, Zhang D (2014) Matrix metalloproteinase-1 (MMP-1) expression in rat spinal cord injury model. Cell Mol Neurobiol 34(8):1151–1163. https://doi.org/10.1007/s10571-014-0090-5
Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, Noble-Haeusslein LJ (2006) Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci 26(39):9841–9850. https://doi.org/10.1523/JNEUROSCI.1993-06.2006
Ogier C, Bernard A, Chollet AM, Le Diguardher T, Hanessian S, Charton G, Khrestchatisky M, Rivera S (2006) Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia 54(4):272–284. https://doi.org/10.1002/glia.20349
Phillips LL, Reeves TM (2001) Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor Neurol Neurosci 19(3–4):213–235
Hayashita-Kinoh H, Kinoh H, Okada A, Komori K, Itoh Y, Chiba T, Kajita M, Yana I, Seiki M (2001) Membrane-type 5 matrix metalloproteinase is expressed in differentiated neurons and regulates axonal growth. Cell Growth Differen 12(11):573–580
Kieseier BC, Pischel H, Neuen-Jacob E, Tourtellotte WW, Hartung HP (2003) ADAM-10 and ADAM-17 in the inflamed human CNS. Glia 42(4):398–405. https://doi.org/10.1002/glia.10226
Bernstein HG, Bukowska A, Krell D, Bogerts B, Ansorge S, Lendeckel U (2003) Comparative localization of ADAMs 10 and 15 in human cerebral cortex normal aging, Alzheimer disease and Down syndrome. J Neurocytol 32(2):153–160
Ortiz RM, Karkkainen I, Huovila AP, Honkaniemi J (2005) ADAM9, ADAM10, and ADAM15 mRNA levels in the rat brain after kainic acid-induced status epilepticus. Brain Res Mol Brain Res 137(1–2):272–275. https://doi.org/10.1016/j.molbrainres.2005.03.008
Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST (2010) Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 166(2):508–521. https://doi.org/10.1016/j.neuroscience.2009.12.061
Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW (2008) Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA 105(49):19520–19525. https://doi.org/10.1073/pnas.0807248105
Reeves TM, Prins ML, Zhu J, Povlishock JT, Phillips LL (2003) Matrix metalloproteinase inhibition alters functional and structural correlates of deafferentation-induced sprouting in the dentate gyrus. J Neurosci 23(32):10182–10189
Harris LKBR, Reeves TM, Phillips LL (2012) Application of zymographic methods to study matrix enzymes following traumatic brain injury. In: Chen JXZ, Xu X-M, Zheng J (eds) Animal models of acute neurological injuries II: injury and mechanistic assessments. Humana Press, New York
DA Reeves TM, Phillips LL (2013) Unmyelinated and myelinated axons exhibit differential injury and treatment responses following traumatic injury. In: Baltan SCS, Matute C, Xi G, Zhang JH (eds) White matter injury in stroke and CNS diseases. Springer Press, New York
Reeves TM, Phillips LL, Povlishock JT (2005) Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 196(1):126–137. https://doi.org/10.1016/j.expneurol.2005.07.014
Trivedi AHJ, Lin Y, Goussev S, Gan J, Topp KS, Noble-Haeusslein LJ (2005) The effects of acute and extended inhibition of matrix metalloproteinases on demyelination and functional recovery after spinal cord injury. Int J Neuroprotect Neuroregen 2:30–38
Levine JM, Cohen ND, Heller M, Fajt VR, Levine GJ, Kerwin SC, Trivedi AA, Fandel TM, Werb Z, Modestino A, Noble-Haeusslein LJ (2014) Efficacy of a metalloproteinase inhibitor in spinal cord injured dogs. PLoS One 9(5):e96408. https://doi.org/10.1371/journal.pone.0096408
Levine JM, Levine GJ, Porter BF, Topp K, Noble-Haeusslein LJ (2011) Naturally occurring disk herniation in dogs: an opportunity for pre-clinical spinal cord injury research. J Neurotrauma 28(4):675–688. https://doi.org/10.1089/neu.2010.1645
Moore SA, Granger N, Olby NJ, Spitzbarth I, Jeffery ND, Tipold A, Nout-Lomas YS, da Costa RC, Stein VM, Noble-Haeusslein LJ, Blight AR, Grossman RG, Basso DM, Levine JM (2017) Targeting translational successes through CANSORT-SCI: using pet dogs to identify effective treatments for spinal cord injury. J Neurotrauma 34(12):2007–2018. https://doi.org/10.1089/neu.2016.4745
Bock P, Spitzbarth I, Haist V, Stein VM, Tipold A, Puff C, Beineke A, Baumgartner W (2013) Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury. Brain Pathol 23(1):82–99. https://doi.org/10.1111/j.1750-3639.2012.00617.x
Levine JM, Ruaux CG, Bergman RL, Coates JR, Steiner JM, Williams DA (2006) Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am J Vet Res 67(2):283–287. https://doi.org/10.2460/ajvr.67.2.283
Levine JM, Cohen ND, Fandel TM, Levine GJ, Mankin J, Griffin JF, Kerwin SC, Boudreau CE, Trivedi A, Noble-Haeusslein LJ (2017) Early Blockade of matrix metalloproteinases in spinal-cord-injured dogs results in a long-term increase in bladder compliance. J Neurotrauma 34(18):2656–2667. https://doi.org/10.1089/neu.2017.5001
Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3(6):422–433. https://doi.org/10.1038/nrc1094
Acknowledgements
We would like to acknowledge the following grant support for this work: Department of Defense grant DOD SCIRP W81XWH-11-1-79 to LJN-H, and National Institutes of Health Grants NS39278 to LJN-H, NS56247 to LP and NS57758 to TR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trivedi, A., Noble-Haeusslein, L.J., Levine, J.M. et al. Matrix metalloproteinase signals following neurotrauma are right on cue. Cell. Mol. Life Sci. 76, 3141–3156 (2019). https://doi.org/10.1007/s00018-019-03176-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03176-4