Abstract
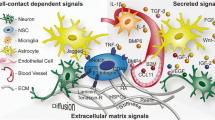
The stem cell niche refers to a specific microenvironment where stem cells proliferate and differentiate to produce new specialized cells throughout an organism’s adulthood. Growth factors are crucial signaling molecules that diffuse through the extracellular space, reach the stem cell niche, and ultimately promote stem cell proliferation and differentiation. However, it is not well known how multiple growth factors, often with antagonistic activities, work together in the stem cell niche to select target stem cell populations and determine stem cell fate. There is accumulating evidence suggesting that extracellular matrix (ECM) molecules play an important role in promoting growth factor access and activity in the stem cell niche. In the adult brain neurogenic zone, where neural stem cells (NSCs) reside, there exist specialized ECM structures, which we have named fractones. The processes of NSC allow them to come into contact with fractones and interact with its individual components, which include heparan sulfate proteoglycans (HSPGs) and laminins. We have demonstrated that fractone-associated HSPGs bind growth factors and regulate NSC proliferation in the neurogenic zone. Moreover, emerging results show that fractones are structurally altered in animal models with autism and adult hydrocephalus, as demonstrated by changes in fractone size, quantity, or HSPG content. Interestingly, ECM structures similar to fractones have been found throughout β-amyloid plaques in the brain of patients with Alzheimer’s disease. Pathological fractones may cause imbalances in growth factor activity and impair neurogenesis, leading to inflammation and disorder. Generally speaking, these stem cell niche structures play a potentially vital role in controlling growth factor activity during both health and disease.






Similar content being viewed by others
References
Avashi S, Srivastava RN, Singh A, Srivastava M (2008) Stem cell: past, present and future—a review article. Intern J Med Update 3(1):22–30
Messina E, De Angelise L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MVG, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A (2004) Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95:911–921
Doetsch J, Garcia-Verdugo JM, Alvarez-Buylla (1997) Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17:5046–5061
Ming GL, Song H (2001) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(687):702
Rojas Rios P, Gonzalez-Reyes A (2014) The plasticity of stem cell niches: general properties behind homeostasis and repair. Stem Cells 32:852–859
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23:609–620
Burgess AW (2015) Growth factors and cytokines. Rev Cell Biol Mol Med 1:104–126
Levi-Montalcini R, Angeletti PU (1964) Hormonal control of the NGF content in the submaxillary glands of mice. Int Ser Monogr Oral Biol 3:129–141
Mercier F, Hatton GI (2004) Meninges and perivasculature as mediators of CNS plasticity. In: Non-neuronal cells in the nervous system: function and dysfunction. Adv Mol Cell Biol 31:215–253
Nakagawa T, Schwartz JP (2004) Expression of neurotrophic factors and cytokines and their receptors on astrocytes in vivo. In: Non-neuronal cells in the nervous system: function and dysfunction. Adv Mol Cell Biol 31:561–273
Ornitz DM, Itoh M (2015) The fibroblast growth factor signaling pathway. Rev Dev Biol 4:215–266
Wachs FP, Winner B, Couillard-Despres S, Shiller T, Aigner R, Winkler J, Bogdahn U, Aigner L (2006) Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J Neuropathol Exp Neurol 65:358–370
Brickman YG, Ford MD, Small DH, Bartlett PF, Nurcombe V (1995) Heparan sulfates mediate the binding of basic fibroblast growth factor to a specific receptor on neural precursor cells. J Biol Chem 270:24941–24948
Gordon MY, Riley GP, Watt SM, Greaves MS (1987) Compartmentalization of a hemopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 326:403–405
Roberts R, Gallagher J, Spooncer E, Alien TD, Bloomfield F, Dexter TM (1988) Heparan-sulfate bound growth factors: a mechanism for stromal cell-mediated hematopoesis. Nature 332:376–378
Rapraeger AC, Guimond S, Krufka A, Olwin BB (1994) Regulation by heparin sulfate in fibroblast growth factor signaling. Methods Enzymol 245:219–240
Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM (1991) Cell surface, heparin-like molecules are required for binding of fibroblast growth factor to its high affinity receptor. Cell 64:841–848
Mercier F, Kitasako JT, Hatton GI (2002) Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188
Douet V, Arikawa-Hirasawa E, Mercier F (2012) Fractone-heparan sulfates mediate BMP-7-inhibition of cell proliferation in the adult subventricular zone. Neurosci Lett 528:120–125
Douet V, Arikawa-Hirasawa E, Mercier F (2013) Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone. Cell Prolif 46:137–145
Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F (2007) Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor FGF-2 from the extracellular milieu. Stem Cells 25:2146–2157
Mercier F, Douet V (2014) Bone morphogenetic protein-4 inhibits adult neurogenesis and is regulated by fractone-heparan sulfates in the subventricular zone. J Chem Neuroanat 57–58:54–61
Clemmons DR (2004) The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest 113:25–27
Grau V, Wilker S, Lips KS, Hartman P, Rose F, Padberg W, Fehrenbach H, Wessler I, Kummer W (2007) Administration of keratinocyte growth factor down regulates the pulmonary capacity of acetylcholine production. Int J Biochem Cell Biol 39:1955–1963
Turnbull AV, Rivier CL (1999) Regulation of the hypothalamic-pituitary-adrenal axis by cytokines actions and mechanisms of actions. Physiol Rev 79:1–71
Yajima Y, Saito T (1984) The effect of epidermal growth factor on cell proliferation and prolactin production by GH3 rat pituitary cells. J Cell Physiol 120:249–256
Thorne RG, Hrabetova S, Nicholson C (2004) Diffusion of epidermal growth factor in the rat brain extracellular space measured by integrative optical imaging. J Neurophysiol 92:3471–3481
Connor B, Dragunow M (1998) The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res Rev 27:1–39
Steed DL (1997) The role of growth factors in wound healing. Surg Clinics 77:575–586
Wahl SM, Wong H, McCartney-Francis (1989) Role of growth factors in inflammation and repair. J Cell Biochem 40:193–199
Kim SH, Turnbull J, Guimond S (2011) Extracellular matrix and cell signaling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol 209:139–151
Eliceri BP (2001) Integrin and growth factor receptor crosstalk. Circ Res 89:1104–1110
Coltrini D, Rusnati M, Zoppetti G, Oreste P, Isacchi A, Caccia P, Bergonzoni L, Presta M (1993) Biochemical bases for interactions of human basic fibroblast growth factor with glycosaminoglycans. New insights from trypsin digestion studies. Eur J Biochem 214:51–58
Farhquar MG (1991) The glomerular basement membrane. In: Hay ED (ed) Cell biology of the extracellular matrix. Plenum Press, New York, pp 365–418
Castro-Munozledo F (2013) Review: corneal epithelial stem cells, their niche and wound healing. Mol Vis 19:1600–1613
Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Sekiguchi K, Reichardt F, Watt FM (2011) The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144:577–589
Kazanis I, ffrench-Constant C (2011) Extracellular matrix and the neural stem cell niche. Dev Neurobiol 71:1006–1017
Mackay-Sim A (2010) Stem cells and their niche in the adult olfactory mucosa. Arch Ital Biol 148:47–58
Mercier F, Kitasako JT, Hatton GI (2003) Fractones and other basal laminae in the hypothalamus. J Comp Neurol 455:324–340
Mercier F, Schnack J, Saint George Chaumet M (2011) Fractones: home and conductors of the neural stem cell niche. In: Seki T et al (eds) Neurogenesis in the adult brain, vol 1. Springer, Japan, pp 109–136
Bifari F, Beto N, Pino A, Kusalo M, Malpeli G, Di Chio M, Bersan E, Amato E, Scarpa A, Kamprepra M, Fumagalli G, Decimo I (2015) Meninges harbor cell-expressing neural precursor markers during development and adulthood. Front Cell Neurosci 9:383
Chyba M, Mercier F, Rader J, Douet V, Arikawa-Hirasawa E, Chow Kwon Y, Kodama R (2011) Dynamic mathematical modeling of cell-fractone interactions. J Math Ind 3:79–88
Mercier F, Mambie S, Hatton GI (2006) Brain macrophages: enigmas and conundrums. In: Dermietzel et al (eds) Blood-brain barriers—from ontogeny to artificial barriers. Wiley-VCH, Weinheim, pp 129–165
Mercier F (2004) Astroglia as a modulation interface between meninges and neurons. In: Hatton GI, Parpura V (eds) Glial/neuronal signaling, chapter 5. Kluwer Publishers, Amsterdam, pp 125–162
Kerever A, Mercier F, Nonaka R, de Vega S, Oda Y, Zalc B, Okada Y, Hottari N, Yamada Y, Arikawa-Hirasawa E (2014) Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res 12:492–505
Das GD, Altman J (1970) Postnatal neurogenesis in the caudate nucleus and nucleus accumbens septi in the rat. Brain Res 21:122–127
Lois C, Alvarez-Buylla A (1994) Long distance neuronal migration in the adult mammalian brain. Science 265:1145–1148
Mercier F, Arikawa-Hirasawa E (2012) Heparan sulfate niche for cell proliferation in the adult brain. Neurosci Lett 510:67–72
Seki T (2002) Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. J Neurosci Res 69:772–783
Seri B, Herrera DG, Gritti A, Ferron S, Collado L, Vescovi A, Garcia-Verdugo JM, Alvarez-Buylla A (2006) Composition and organization of the SCZ. A large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex 16:103–111
Mandelbrot BB (1983) The fractal geometry of nature. WH Freeman, San Francisco
Ling EA (1998) Origin, nature and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech 41:43–56
Hada SH, Habuchi H, Kariya Y, Itoh N, Reddi AH, Kimata K (2004) Characterization of growth factor binding structures in heparin/heparan sulfate using an octasaccharide library. J Biol Chem 279:12346–12354
Brightman MW (2002) The brain’s interstitial clefts and their glial walls. J Neurocytol 31(2002):569–603
Ishihara M, Guo Y, Wei Z, Yang Z, Swiedler SJ, Orellana A, Hirsberg CB (1993) Regulation of biosynthesis of the basis fibroblast growth factor binding domains of heparan sulfate by heparan sulfate-N-deacetylase/N-sulfotransferase expression. J Biol Chem 268:20091–20095
Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Gotz M (2008) Ault neurogenesis requires smad4-mediated bone morphogenetic protein signaling in stem cells. J Neurosci 28:434–446
Dierker T, Dreier R, Peterson A, Bordych C, Grobe K (2009) Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem 284:8013–8022
Gordts PL, Esko JD (2015) Heparan sulfate proteoglycans fine-tune macrophage inflammation via IFN-gamma. Cytokine 72:118–119
Khan SA, Nelson MS, Pan C, Gaffney PM, Gupta P (2008) Endogenous heparan sulfate and heparin modulate bone morphogenetic protein-4 signaling and activity. Am J Physiol Cell Physiol 294:1387–1397
Kishimoto S, Nakamura S, Hattori H, Nakamura SI, Oonuma F, Kanatani Y, Tanaka Y, Mori Y, Harada Y, Tagawa M, Ishihara M (2009) Human stem cell factor (SCF) is a heparin-binding cytokine. J Biochem 145:275–278
Lyon M, Rushton G, Gallagher JT (1997) The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform specific. J Biol Chem 18:18000–18006
Thorne BA, Plowman GD (1994) The heparin-binding domain of amphiregulin necessitates the precursor pro-region for growth factor secretion. Mol Cell Biol 14:1635–1646
Johanson C, McMillan P, Tavares R, Spangenberger A, Duncan J, Silverberg G, Stopa E (2004) Homeostatic capabilities of the choroid plexus epithelium in Alzheimer’s disease. Cerebrospinal Fluid Res 1:3
Stopa EG, Berzin TM, Kim S, Song P, Kuo-Leblnc V, Rodriguez-Wolf M, Johanson CE (2001) Human choroid plexus growth factors: what are the implications for CSF dynamics in Alzheimer’s disease? Exp Neurol 167:40–47
Choe Y, Kozlova A, Graf D, Pleasure SJ (2013) Bone morphogenetic protein signaling is a major determinant of dentate development. J Neurosci 16:6667–6675
Bifari F, Decimo I, Chimulera C, Bersan E, Malpelli G, Johansson J, Lisi V, Bonetti B, Fumagalli G, Pizzolo G, Krampera M (2009) Novel stem/progenitor cells with neuronal differentiation potential reside in the leptomeningeal niche. J Cell Mol Med 9B:3195–3208
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske D, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J (2015) Structural and functional features of central nervous system lymphatics. Nature 523:531–533
Mercier F, Weatherby TM, Harttine DK (2013) Meningeal-like organization of neural tissue sin calanoid copepods. J Comp Neurol 521:760–790
Siegenthaler JA, Pleasure SJ (2011) We have got you covered: how the meninges control brain development. Curr Opin Genet Dev 21:249–255
Mercier F, Cho-Kown Y, Douet D (2012) Hippocampus/amygdala alterations, loss of heparan sulfates, fractones and ventricle wall reduction in adult BTBR T+tf/J mice, animal model for autism. Neurosci Lett 506:208–213
Mercier F, Cho-Kwon Y, Kodama R (2011) Meningeal/vascular alterations and loss of extracellular matrix in the neurogenic zone of adult BTBR T+tf/J mice, animal model for autism. Neurosci Lett 498:173–178
Wahlsten D, Metten P, Crabbe JC (2003) Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T+tf/J has severely reduced hippocampal commissure and absent corpus callosum. Brain Res 971:44–57
Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro LP, Barbaro JR, West LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN (2007) Mouse behavioural tasks relevant to autism: phenotype of 10 inbred mice. Behav Brian Res 176:4–20
Campos-Ordonez T, Herranz-Perez V, Chaichana KL, Riccon-Torroella J, Garcia-Verdugo JM, Quinines-Hinojosa A, Gonzalez-Perez O (2014) Long-term hydrocephalus alters the cytoarchitecture of the adult subventricular zone. Exp Neurol 261:236–244
Palu E, Liesi P (2002) Differential distribution of laminins in Alzheimer disease and normal human brain tissue. J Neurosci Res 69:243–256
Yasuoka K, Hirata K, Kuraoka A, Kawabuchi M (2004) Expression of amyloid precursor protein-like molecule in astroglial cells of he subventricular zone and rostral migratory stream of the adult rat forebrain. J Anat 205:135–146
Leveugle B, Scanameo A, Ding W, Fillit H (1994) Binding of heparan sulfate glycosaminoglycan to beta-amyloid peptide: inhibition by potentially therapeutic polysulfated compounds. NeuroReport 5:1389–1392
Scholefield Z, Yates EA, Wayne G, Amour A, McDowell W, Turnbull JE (2003) Heparan sulfate regulate amyloid precursor protein processing by BACE1, the Alzheimer’s β-secretase. J Cell Biol 163:97–107
Homes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotsbauer PT, Miller TM, Papy-Garcia D, Diamnon MI (2013) Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci 110:E3138–E3147
Irie F, Badie-Mahdavi H, Yamaguchi Y (2012) Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc Natl Aced Sci 109:5052–5056
Campanella GSV, Em Lee, Sun J, Luster AD (2003) CXCR3 and heparin binding sites of the chemokine IP-10 (CXCL10). J Biol Chem 278:17066–17074
Kerever Yamada T, Suzuki Y, Mercier F, Arikawa-Hirasawa E (2015) Fractone aging in the subventricular zone of the lateral ventricle. J Chem Neuroanat 66–67:52–60
Gonzalez-Perez O, Alvarez-Buylla A (2011) Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev 67:147–156
Relucio J, Menezes M, Miyagoe-Suzuki MJ, Takeda S, Colognato H (2012) Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte precursor survival in the subventricular zone. Glia 60:1451–1467
El Waly B, Macchi M, Cayre M, Durbec P (2014) Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci 8:145
Winkler S, Stahl RC, Carey DJ, Bansal R (2002) Syndecan-3 and perlecan are differentially expressed by progenitor and mature oligodendrocytes and accumulate in the extracellular matrix. J Neurosci Res 69:477–487
Pye DA, Vived RR, Turnbull JE, Hyde P, Gallagher JT (1998) Heparan sulfate oligosaccharide require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J Biol Chem 273:22936–22942
Sasaki N, Higashi N, Taka T, Nakajima M, Irimura T (2004) Cell surface localization of heparanase on macrophages regulates degradation of extracellular matrix heparan sulfate. J Immunol 172:3830–3835
Edwards IJ, Xu H, Obonuke JC, Goldberg IJ, Wagner WD (1995) Differentiated macrophages synthetize a heparan sulfate proteoglycan and an oversulfated chondrotine sulfate proteoglycan that bind lipoprotein lipase. Arterioscler Thromb Vasc Biol 15:400–409
Zhang GL, Zhang X, Wanfg XM, Li JP (2014) Towards understanding the role of heparan sulfate proteoglycans in Alzheimer’s disease. BioMed Res Int. doi:10.1155/2014/516028
Perez-Martin M, Grondona JM, Cifuentes M, Perez-Figares JM, Jimenez JA, Fernandez-Llebrez P (2000) Ependymal explants from the lateral ventricle of the adult bovine brain: a model system for morphological and functional studies of the ependyma. Cell Tissue Res 300:11–19
Mercier F, Hatton GI (2000) Immunocytochemical basis for a meningeo-glial network. J Comp Neurol 420:445–465
Acknowledgments
I thank C Alan Titchenal, Harry B Davis and Albert HW Jiang for proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mercier, F. Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 73, 4661–4674 (2016). https://doi.org/10.1007/s00018-016-2314-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2314-y