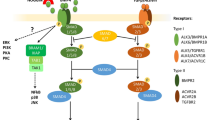
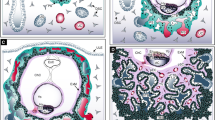
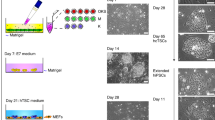
Abstract
The critical role of the placenta in supporting a healthy pregnancy is mostly ensured by the extraembryonic trophoblast lineage that acts as the interface between the maternal and the foetal compartments. The diverse trophoblast cell subtypes that form the placenta originate from a single layer of stem cells that emerge from the embryo when the earliest cell fate decisions are occurring. Recent studies show that these trophoblast stem cells exhibit extensive plasticity as they are capable of differentiating down multiple pathways and are easily converted into embryonic stem cells in vitro. In this review, we discuss current knowledge of the mechanisms and control of the epigenesis of mouse trophoblast stem cells through a comparison with the corresponding mechanisms in pluripotent embryonic stem cells. To illustrate some of the more striking manifestations of the epigenetic plasticity of mouse trophoblast stem cells, we discuss them within the context of two paradigms of epigenetic regulation of gene expression: the imprinted gene expression of specific loci and the process of X-chromosome inactivation.



Similar content being viewed by others
References
Rossant J, Tam PP (2009) Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136(5):701–713
Saiz N, Plusa B (2013) Early cell fate decisions in the mouse embryo. Reproduction 145(3):R65–R80
Cross JC et al (2002) Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol 187(1–2):207–212
Georgiades P, Ferguson-Smith AC, Burton GJ (2002) Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23(1):3–19
John R, Hemberger M (2012) A placenta for life. Reprod Biomed Online 25(1):5–11
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292(5819):154–156
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78(12):7634–7638
Tanaka S et al (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science 282(5396):2072–2075
Efthymiou AG et al (2014) Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther 14(9):1333–1344
Copp AJ (1979) Interaction between inner cell mass and trophectoderm of the mouse blastocyst. II. The fate of the polar trophectoderm. J Embryol Exp Morphol 51:109–120
Hemberger M, Hughes M, Cross JC (2004) Trophoblast stem cells differentiate in vitro into invasive trophoblast giant cells. Dev Biol 271(2):362–371
Hemberger M et al (2001) Genetic and developmental analysis of X-inactivation in interspecific hybrid mice suggests a role for the Y chromosome in placental dysplasia. Genetics 157(1):341–348
Maltepe E, Bakardjiev AI, Fisher SJ (2010) The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest 120(4):1016–1025
Rugg-Gunn PJ et al (2012) Cell-surface proteomics identifies lineage-specific markers of embryo-derived stem cells. Dev Cell 22(4):887–901
Simmons DG, Fortier AL, Cross JC (2007) Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol 304(2):567–578
Logan PC, Mitchell MD, Lobie PE (2013) DNA methyltransferases and TETs in the regulation of differentiation and invasiveness of extra-villous trophoblasts. Front Genet 4:265
Perry JK et al (2010) Regulation of invasive growth: similar epigenetic mechanisms underpin tumour progression and implantation in human pregnancy. Clin Sci 118:451–457
Richard G, Puisieux A, Caramel J (2014) Antagonistic functions of EMT-inducers in melanoma development: implications for cancer cell plasticity. Cancer Cell Microenviron 1:e61
Nichols J et al (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95(3):379–391
Strumpf D et al (2005) Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132(9):2093–2102
Albert M, Peters AH (2009) Genetic and epigenetic control of early mouse development. Curr Opin Genet Dev 19(2):113–121
Niwa H et al (2005) Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123(5):917–929
Zernicka-Goetz M, Morris SA, Bruce AW (2009) Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet 10(7):467–477
Nishioka N et al (2008) Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech Dev 125(3–4):270–283
Nishioka N et al (2009) The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16(3):398–410
Ralston A, Rossant J (2008) Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev Biol 313(2):614–629
Stephenson RO, Yamanaka Y, Rossant J (2010) Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development 137(20):3383–3391
Kondratiuk I et al (2012) Delay of polarization event increases the number of Cdx2-positive blastomeres in mouse embryo. Dev Biol 368(1):54–62
Jedrusik A et al (2008) Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev 22(19):2692–2706
Gardner RL, Papaioannou VE, Barton SC (1973) Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. J Embryol Exp Morphol 30(3):561–572
Rossant J, Tamura-Lis W (1981) Effect of culture conditions on diploid to giant-cell transformation in postimplantation mouse trophoblast. J Embryol Exp Morphol 62:217–227
Ilgren EB (1981) On the control of the trophoblastic giant-cell transformation in the mouse: homotypic cellular interactions and polyploidy. J Embryol Exp Morphol 62:183–202
Arman E et al (1998) Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA 95(9):5082–5087
Haffner-Krausz R et al (1999) Expression of Fgfr2 in the early mouse embryo indicates its involvement in preimplantation development. Mech Dev 85(1–2):167–172
Niswander L, Martin GR (1992) Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 114(3):755–768
Feldman B et al (1995) Requirement of FGF-4 for postimplantation mouse development. Science 267(5195):246–249
Bedzhov I et al (2014) Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Phil Trans R Soc London B Biol Sci 369:20130538. doi:10.1098/rstb.2013.0538
Guzman-Ayala M et al (2004) Nodal protein processing and fibroblast growth factor 4 synergize to maintain a trophoblast stem cell microenvironment. Proc Natl Acad Sci USA 101(44):15656–15660
Lu CW et al (2008) Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet 40(7):921–926
Rossant J (2001) Stem cells from the mammalian blastocyst. Stem Cells 19(6):477–482
Saba-El-Leil MK et al (2003) An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep 4(10):964–968
Himeno E, Tanaka S, Kunath T (2008) Isolation and manipulation of mouse trophoblast stem cells. Curr Protoc Stem Cell Biol Chapter 1:Unit 1E.4
Kubaczka C et al (2014) Derivation and maintenance of murine trophoblast stem cells under defined conditions. Stem Cell Rep 2(2):232–242
Krueger WH et al (2010) Natural and artificial routes to pluripotency. Int J Dev Biol 54(11–12):1545–1564
Soza-Ried J, Fisher AG (2012) Reprogramming somatic cells towards pluripotency by cellular fusion. Curr Opin Genet Dev 22(5):459–465
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Ogawa H et al (2015) Deficiency of genomic reprogramming in trophoblast stem cells following nuclear transfer. Cell Reprogram 17(2):115–123
Santos J et al (2010) Differences in the epigenetic and reprogramming properties of pluripotent and extra-embryonic stem cells implicate chromatin remodelling as an important early event in the developing mouse embryo. Epigenetics Chromatin 3:1
Kuckenberg P et al (2011) Lineage conversion of murine extraembryonic trophoblast stem cells to pluripotent stem cells. Mol Cell Biol 31(8):1748–1756
Wu T et al (2011) Reprogramming of trophoblast stem cells into pluripotent stem cells by Oct4. Stem Cells 29(5):755–763
Schenke-Layland K et al (2007) Collagen IV induces trophoectoderm differentiation of mouse embryonic stem cells. Stem Cells 25(6):1529–1538
Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24(4):372–376
Cambuli F et al (2014) Epigenetic memory of the first cell fate decision prevents complete ES cell reprogramming into trophoblast. Nat Commun 5:5538
Meshorer E, Misteli T (2006) Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol 7(7):540–546
Meshorer E et al (2006) Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell 10(1):105–116
Efroni S et al (2008) Global transcription in pluripotent embryonic stem cells. Cell Stem Cell 2(5):437–447
Ahmed K et al (2010) Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo. PLoS One 5(5):e10531
Rugg-Gunn PJ et al (2010) Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc Natl Acad Sci USA 107(24):10783–10790
Saha B et al (2013) EED and KDM6B coordinate the first mammalian cell lineage commitment to ensure embryo implantation. Mol Cell Biol 33(14):2691–2705
Dahl JA et al (2010) Histone H3 lysine 27 methylation asymmetry on developmentally-regulated promoters distinguish the first two lineages in mouse preimplantation embryos. PLoS One 5(2):e9150
Alder O et al (2010) Ring1B and Suv39h1 delineate distinct chromatin states at bivalent genes during early mouse lineage commitment. Development 137(15):2483–2492
Bernstein BE et al (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125(2):315–326
Chapman V et al (1984) Cell lineage-specific undermethylation of mouse repetitive DNA. Nature 307(5948):284–286
Rossant J et al (1986) Undermethylation of structural gene sequences in extraembryonic lineages of the mouse. Dev Biol 117(2):567–573
Santos F et al (2002) Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 241(1):172–182
Senner CE et al (2012) DNA methylation profiles define stem cell identity and reveal a tight embryonic-extraembryonic lineage boundary. Stem Cells 30(12):2732–2745
Farthing CR et al (2008) Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS Genet 4(6):e1000116
Hattori N et al (2007) Epigenetic regulation of Nanog gene in embryonic stem and trophoblast stem cells. Genes Cells 12(3):387–396
Hattori N et al (2004) Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J Biol Chem 279(17):17063–17069
Nakanishi MO et al (2012) Trophoblast-specific DNA methylation occurs after the segregation of the trophectoderm and inner cell mass in the mouse periimplantation embryo. Epigenetics 7(2):173–182
Wang K et al (2010) Brg1 is required for Cdx2-mediated repression of Oct4 expression in mouse blastocysts. PLoS One 5(5):e10622
Donnison M et al (2005) Loss of the extraembryonic ectoderm in Elf5 mutants leads to defects in embryonic patterning. Development 132(10):2299–2308
Ng RK et al (2008) Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat Cell Biol 10(11):1280–1290
Carey TS et al (2014) Transcriptional reprogramming and chromatin remodeling accompanies Oct4 and Nanog silencing in mouse trophoblast lineage. Stem Cells Dev 23(3):219–229
Yuan P et al (2009) Eset partners with Oct4 to restrict extraembryonic trophoblast lineage potential in embryonic stem cells. Genes Dev 23(21):2507–2520
Sakaue M et al (2010) DNA methylation is dispensable for the growth and survival of the extraembryonic lineages. Curr Biol 20(16):1452–1457
Kidder BL, Palmer S (2012) HDAC1 regulates pluripotency and lineage specific transcriptional networks in embryonic and trophoblast stem cells. Nucleic Acids Res 40(7):2925–2939
Zhu D et al (2014) Lysine-specific demethylase 1 regulates differentiation onset and migration of trophoblast stem cells. Nat Commun 5:3174
Barlow DP (2011) Genomic imprinting: a mammalian epigenetic discovery model. Annu Rev Genet 45:379–403
Bartolomei MS1, Ferguson-Smith AC (2011) Mammalian genomic imprinting. Cold Spring Harb Perspect Biol 3(7):a002592
Duffie R, Bourc’his D (2013) Parental epigenetic asymmetry in mammals. Curr Top Dev Biol 104:293–328
Mohammad F, Mondal T, Kanduri C (2009) Epigenetics of imprinted long noncoding RNAs. Epigenetics 4(5):277–286
Santoro F, Barlow DP (2011) Developmental control of imprinted expression by macro non-coding RNAs. Semin Cell Dev Biol 22(4):328–335
Ito M et al (2015) A trans-homologue interaction between reciprocally imprinted miR-127 and Rtl1 regulates placenta development. Development 142(14):2425–2430
Hanna JH, Saha K, Jaenisch R (2010) Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 143(4):508–525
Bressan FF et al (2009) Unearthing the roles of imprinted genes in the placenta. Placenta 30(10):823–834
Hudson QJ et al (2010) Genomic imprinting mechanisms in embryonic and extraembryonic mouse tissues. Heredity (Edinb) 105(1):45–56
Radford EJ, Ferron SR, Ferguson-Smith AC (2011) Genomic imprinting as an adaptative model of developmental plasticity. FEBS Lett 585(13):2059–2066
Li L et al (1999) Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 284(5412):330–333
Constancia M et al (2002) Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417(6892):945–948
Lefebvre L et al (1998) Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet 20(2):163–169
Weaver JR, Susiarjo M, Bartolomei MS (2009) Imprinting and epigenetic changes in the early embryo. Mamm Genome 20(9–10):532–543
Lewis A et al (2006) Epigenetic dynamics of the Kcnq1 imprinted domain in the early embryo. Development 133(21):4203–4210
Pandey RR et al (2008) Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 32(2):232–246
Murakami K, Oshimura M, Kugoh H (2007) Suggestive evidence for chromosomal localization of non-coding RNA from imprinted LIT1. J Hum Genet 52(11):926–933
Terranova R et al (2008) Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Dev Cell 15(5):668–679
Nagano T et al (2008) The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322(5908):1717–1720
Gendrel AV, Heard E (2014) Noncoding RNAs and epigenetic mechanisms during X-chromosome inactivation. Annu Rev Cell Dev Biol 30:561–580
Morey C, Avner P (2011) The demoiselle of X-inactivation: 50 years old and as trendy and mesmerising as ever. PLoS Genet 7(7):e1002212
Barakat TS, Gribnau J (2012) X chromosome inactivation in the cycle of life. Development 139(12):2085–2089
Deuve JL, Avner P (2011) The coupling of X-chromosome inactivation to pluripotency. Annu Rev Cell Dev Biol 27:611–629
Mak W et al (2004) Reactivation of the paternal X chromosome in early mouse embryos. Science 303(5658):666–669
Okamoto I et al (2004) Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303(5658):644–649
Patrat C et al (2009) Dynamic changes in paternal X-chromosome activity during imprinted X-chromosome inactivation in mice. Proc Natl Acad Sci USA 106(13):5198–5203
Sado T et al (2000) X inactivation in the mouse embryo deficient for Dnmt1: distinct effect of hypomethylation on imprinted and random X inactivation. Dev Biol 225(2):294–303
Marahrens Y et al (1997) Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev 11(2):156–166
Senner CE et al (2011) Disruption of a conserved region of Xist exon 1 impairs Xist RNA localisation and X-linked gene silencing during random and imprinted X chromosome inactivation. Development 138(8):1541–1550
Kalantry S et al (2009) Evidence of Xist RNA-independent initiation of mouse imprinted X-chromosome inactivation. Nature 460(7255):647–651
Hoki Y et al (2011) Incomplete X-inactivation initiated by a hypomorphic Xist allele in the mouse. Development 138(13):2649–2659
Papaioannou VE, West JD (1981) Relationship between the parental origin of the X chromosomes, embryonic cell lineage and X chromosome expression in mice. Genet Res 37(2):183–197
Kay GF et al (1994) Imprinting and X chromosome counting mechanisms determine Xist expression in early mouse development. Cell 77(5):639–650
Nesterova TB et al (2001) Loss of Xist imprinting in diploid parthenogenetic preimplantation embryos. Dev Biol 235(2):343–350
Goto Y, Takagi N (2000) Maternally inherited X chromosome is not inactivated in mouse blastocysts due to parental imprinting. Chromosome Res 8(2):101–109
Goto Y, Takagi N (1998) Tetraploid embryos rescue embryonic lethality caused by an additional maternally inherited X chromosome in the mouse. Development 125(17):3353–3363
Okamoto I, Tan S, Takagi N (2000) X-chromosome inactivation in XX androgenetic mouse embryos surviving implantation. Development 127(19):4137–4145
Shao C, Takagi N (1990) An extra maternally derived X chromosome is deleterious to early mouse development. Development 110(3):969–975
Tada T, Takagi N, Adler ID (1993) Parental imprinting on the mouse X chromosome: effects on the early development of X0, XXY and XXX embryos. Genet Res 62(2):139–148
Merzouk S et al (2014) Lineage-specific regulation of imprinted X inactivation in extraembryonic endoderm stem cells. Epigenetics Chromatin 7:11
Berletch JB et al (2015) Escape from X inactivation varies in mouse tissues. PLoS Genet 11(3):e1005079
Calabrese JM et al (2012) Site-specific silencing of regulatory elements as a mechanism of X inactivation. Cell 151(5):951–963
Yang F et al (2010) Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res 20(5):614–622
Corbel C et al (2013) Unusual chromatin status and organization of the inactive X chromosome in murine trophoblast giant cells. Development 140(4):861–872
Hadjantonakis AK et al (2001) An X-linked GFP transgene reveals unexpected paternal X-chromosome activity in trophoblastic giant cells of the mouse placenta. Genesis 29(3):133–140
Tam PP, Williams EA, Tan SS (1994) Expression of an X-linked HMG-lacZ transgene in mouse embryos: implication of chromosomal imprinting and lineage-specific X-chromosome activity. Dev Genet 15(6):491–503
Tan SS, Williams EA, Tam PP (1993) X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat Genet 3(2):170–174
Dubois A et al (2014) Spontaneous reactivation of clusters of X-linked genes is associated with the plasticity of X-inactivation in mouse trophoblast stem cells. Stem Cells 32(2):377–390
Mugford JW, Yee D, Magnuson T (2012) Failure of extra-embryonic progenitor maintenance in the absence of dosage compensation. Development 139(12):2130–2138
Hemberger M (2002) The role of the X chromosome in mammalian extra embryonic development. Cytogenet Genome Res 99(1–4):210–217
Guttman M et al (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477(7364):295–300
Flynn RA, Chang HY (2014) Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell 14(6):752–761
Furukawa S, Kuroda Y, Sugiyama A (2014) A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol 27(1):11–18
Knox K, Baker JC (2008) Genomic evolution of the placenta using co-option and duplication and divergence. Genome Res 18(5):695–705
Chuong EB (2013) Retroviruses facilitate the rapid evolution of the mammalian placenta. BioEssays 35(10):853–861
Hemberger M (2010) Genetic-epigenetic intersection in trophoblast differentiation: implications for extraembryonic tissue function. Epigenetics 5(1):24–29
Chuong EB et al (2013) Endogenous retroviruses function as species-specific enhancer elements in the placenta. Nat Genet 45(3):325–329
Acknowledgments
We apologise to the authors who have contributed to related studies or aspects of trophoblast stem cell plasticity that could not be addressed here due to the format restrictions of the review. We thank Dr. Graham Hayhurst for critical reading of the manuscript. J.P. was supported by a doctoral fellowship from the Région Ile-de-France (DIM-StemPôle), and by a grant from the REVIVE Labex. CM is supported on a permanent basis by the French National Institute for Scientific and Medical Research (INSERM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prudhomme, J., Morey, C. Epigenesis and plasticity of mouse trophoblast stem cells. Cell. Mol. Life Sci. 73, 757–774 (2016). https://doi.org/10.1007/s00018-015-2086-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-2086-9