Abstract
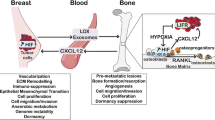
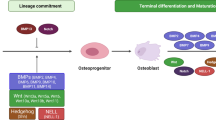
The bone is a complex connective tissue composed of many different cell types such as osteoblasts, osteoclasts, chondrocytes, mesenchymal stem/progenitor cells, hematopoietic cells and endothelial cells, among others. The interaction between them is finely balanced through the processes of bone formation and bone remodeling, which regulates the production and biological activity of many soluble factors and extracellular matrix components needed to maintain the bone homeostasis in terms of cell proliferation, differentiation and apoptosis. Osteosarcoma (OS) emerges in this complex environment as a result of poorly defined oncogenic events arising in osteogenic lineage precursors. Increasing evidence supports that similar to normal development, the bone microenvironment (BME) underlies OS initiation and progression. Here, we recapitulate the physiological processes that regulate bone homeostasis and review the current knowledge about how OS cells and BME communicate and interact, describing how these interactions affect OS cell growth, metastasis, cancer stem cell fate and therapy outcome.



Similar content being viewed by others
Abbreviations
- AKT:
-
V-Akt murine thymoma viral oncogene homolog
- ALDH:
-
Aldehyde dehydrogenase
- BM:
-
Bone marrow
- BMP:
-
Bone morphogenic proteins
- CCL:
-
Chemokine (C–C Motif) ligands
- CSC:
-
Cancer stem cells
- CXCL:
-
Chemokine (C–X–C Motif) ligands
- DKK:
-
Dickkopf proteins
- ECM:
-
Extracellular matrix
- EDN1:
-
Endothelin 1
- EMV:
-
Extracellular membrane vesicles
- EPH:
-
Erythropoietin-producing hepatoma
- ERK:
-
Extracellular signal-related kinases
- FGF:
-
Fibroblast growth factors
- GLI:
-
Glioma-associated oncogene
- GH:
-
Growth hormone
- GP:
-
Growth plate
- HES:
-
Hairy and enhancer of split
- HH:
-
Hedgehog proteins
- HIF:
-
Hypoxia-inducible factors
- IGF:
-
Insulin-like growth factors
- IHH:
-
Indian hedgehog
- IL:
-
Interleukin
- MAPK:
-
Mitogen-activated protein kinases
- MCT:
-
Monocarboxylate transporter
- miRs:
-
MicroRNAs
- MMP:
-
Matrix metalloproteinases
- MSC:
-
Mesenchymal stem/progenitor cells
- mTOR:
-
Mammalian target of rapamycin
- NFkB:
-
Nuclear factor kB
- OPG:
-
Osteoprotegerin
- OS:
-
Osteosarcoma
- PDGF:
-
Platelet-derived growth factor
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PTHrP:
-
Parathyroid hormone-related peptide
- RANK:
-
Receptor activator of nuclear factor kappa B
- RANKL:
-
RANK ligand
- RB:
-
Retinoblastoma
- SOX2:
-
Sex-determining region Y-box 2
- STAT3:
-
Signal transducer and activator of transcription 3
- TGFα/β:
-
Transforming growth factor α/β
- VEGF:
-
Vascular endothelial growth factors
- WIF1:
-
WNT inhibitory factor 1
- WNT:
-
Wingless-type MMTV integration site family
- YAP1:
-
Yes-associated protein 1
References
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423(6937):332–336
Burdan F, Szumilo J, Korobowicz A, Farooquee R, Patel S, Patel A, Dave A, Szumilo M, Solecki M, Klepacz R, Dudka J (2009) Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol 47(1):5–16
Overholtzer M, Rao PH, Favis R, Lu XY, Elowitz MB, Barany F, Ladanyi M, Gorlick R, Levine AJ (2003) The presence of p53 mutations in human osteosarcomas correlates with high levels of genomic instability. Proc Natl Acad Sci USA 100(20):11547–11552
Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, Yamamuro T (1994) Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res 54(11):3042–3048
Mutsaers AJ, Walkley CR (2014) Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone 62:56–63
Rodriguez R, Garcia-Castro J, Trigueros C, Garcia Arranz M, Menendez P (2012) Multipotent mesenchymal stromal cells: clinical applications and cancer modeling. Adv Exp Med Biol 741:187–205
Rodriguez R, Rubio R, Menendez P (2012) Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res 22(1):62–77
Rubio R, Gutierrez-Aranda I, Saez-Castillo AI, Labarga A, Rosu-Myles M, Gonzalez-Garcia S, Toribio ML, Menendez P, Rodriguez R (2013) The differentiation stage of p53-Rb-deficient bone marrow mesenchymal stem cells imposes the phenotype of in vivo sarcoma development. Oncogene 32(41):4970–4980
Xiao W, Mohseny AB, Hogendoorn PC, Cleton-Jansen AM (2013) Mesenchymal stem cell transformation and sarcoma genesis. Clin Sarcoma Res 3(1):10
Rubio R, Garcia-Castro J, Gutierrez-Aranda I, Paramio J, Santos M, Catalina P, Leone PE, Menendez P, Rodriguez R (2010) Deficiency in p53 but not retinoblastoma induces the transformation of mesenchymal stem cells in vitro and initiates leiomyosarcoma in vivo. Cancer Res 70(10):4185–4194
Rubio R, Abarrategi A, Garcia-Castro J, Martinez-Cruzado L, Suarez C, Tornin J, Santos L, Astudillo A, Colmenero I, Mulero F, Rosu-Myles M, Menendez P, Rodriguez R (2014) Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. Stem Cells 32(5):1136–1148
Richardson RB (2014) Age-specific bone tumour incidence rates are governed by stem cell exhaustion influencing the supply and demand of progenitor cells. Mech Ageing Dev 139:31–40
Kirpensteijn J, Timmermans-Sprang EP, van Garderen E, Rutteman GR, Lantinga-van Leeuwen IS, Mol JA (2002) Growth hormone gene expression in canine normal growth plates and spontaneous osteosarcoma. Mol Cell Endocrinol 197(1–2):179–185
Robson H, Siebler T, Shalet SM, Williams GR (2002) Interactions between GH, IGF-I, glucocorticoids, and thyroid hormones during skeletal growth. Pediatr Res 52(2):137–147
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D (2012) HMGB1 promotes drug resistance in osteosarcoma. Cancer Res 72(1):230–238
Ek ET, Dass CR, Contreras KG, Choong PF (2007) Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumor activities of pigment epithelium-derived factor. Clin Exp Metastasis 24(2):93–106
Theriault RL, Theriault RL (2012) Biology of bone metastases. Cancer Control 19(2):92–101
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473(2):139–146
Broadhead ML, Clark JC, Myers DE, Dass CR, Choong PF (2011) The molecular pathogenesis of osteosarcoma: a review. Sarcoma 2011:959248
Miyamoto N, Higuchi Y, Mori K, Ito M, Tsurudome M, Nishio M, Yamada H, Sudo A, Kato K, Uchida A, Ito Y (2002) Human osteosarcoma-derived cell lines produce soluble factor(s) that induces differentiation of blood monocytes to osteoclast-like cells. Int Immunopharmacol 2(1):25–38
Kingsley LA, Fournier PG, Chirgwin JM, Guise TA (2007) Molecular biology of bone metastasis. Mol Cancer Ther 6(10):2609–2617
Kuchimaru T, Hoshino T, Aikawa T, Yasuda H, Kobayashi T, Kadonosono T, Kizaka-Kondoh S (2014) Bone resorption facilitates osteoblastic bone metastatic colonization by cooperation of insulin-like growth factor and hypoxia. Cancer Sci 105(5):553–559
Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V, Blanchard F, Gouin F, Pitard B, Heymann D, Redini F (2007) Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the “vicious cycle” between tumor cell proliferation and bone resorption. Cancer Res 67(15):7308–7318
Zeng W, Wan R, Zheng Y, Singh SR, Wei Y (2011) Hypoxia, stem cells and bone tumor. Cancer Lett 313(2):129–136
Avnet S, Longhi A, Salerno M, Halleen JM, Perut F, Granchi D, Ferrari S, Bertoni F, Giunti A, Baldini N (2008) Increased osteoclast activity is associated with aggressiveness of osteosarcoma. Int J Oncol 33(6):1231–1238
Costa-Rodrigues J, Teixeira CA, Fernandes MH (2011) Paracrine-mediated osteoclastogenesis by the osteosarcoma MG63 cell line: is RANKL/RANK signalling really important? Clin Exp Metastasis 28(6):505–514
Itoh K, Udagawa N, Matsuzaki K, Takami M, Amano H, Shinki T, Ueno Y, Takahashi N, Suda T (2000) Importance of membrane- or matrix-associated forms of M-CSF and RANKL/ODF in osteoclastogenesis supported by SaOS-4/3 cells expressing recombinant PTH/PTHrP receptors. J Bone Miner Res 15(9):1766–1775
Kinpara K, Mogi M, Kuzushima M, Togari A (2000) Osteoclast differentiation factor in human osteosarcoma cell line. J Immunoassay 21(4):327–340
Costa-Rodrigues J, Fernandes A, Fernandes MH (2011) Reciprocal osteoblastic and osteoclastic modulation in co-cultured MG63 osteosarcoma cells and human osteoclast precursors. J Cell Biochem 112(12):3704–3713
Lee JA, Jung JS, Kim DH, Lim JS, Kim MS, Kong CB, Song WS, Cho WH, Jeon DG, Lee SY, Koh JS (2011) RANKL expression is related to treatment outcome of patients with localized, high-grade osteosarcoma. Pediatr Blood Cancer 56(5):738–743
Rousseau J, Escriou V, Lamoureux F, Brion R, Chesneau J, Battaglia S, Amiaud J, Scherman D, Heymann D, Redini F, Trichet V (2011) Formulated siRNAs targeting Rankl prevent osteolysis and enhance chemotherapeutic response in osteosarcoma models. J Bone Miner Res 26(10):2452–2462
Moriceau G, Ory B, Gobin B, Verrecchia F, Gouin F, Blanchard F, Redini F, Heymann D (2010) Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des 16(27):2981–2987
Heymann D, Ory B, Blanchard F, Heymann MF, Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F, Redini F (2005) Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone 37(1):74–86
Lamoureux F, Moriceau G, Picarda G, Rousseau J, Trichet V, Redini F (2010) Regulation of osteoprotegerin pro- or anti-tumoral activity by bone tumor microenvironment. Biochim Biophys Acta 1805(1):17–24
Picarda G, Trichet V, Teletchea S, Heymann D, Redini F (2012) TRAIL receptor signaling and therapeutic option in bone tumors: the trap of the bone microenvironment. Am J Cancer Res 2(1):45–64
Lamoureux F, Picarda G, Rousseau J, Gourden C, Battaglia S, Charrier C, Pitard B, Heymann D, Redini F (2008) Therapeutic efficacy of soluble receptor activator of nuclear factor-kappa B-Fc delivered by nonviral gene transfer in a mouse model of osteolytic osteosarcoma. Mol Cancer Ther 7(10):3389–3398
Cathomas R, Rothermundt C, Bode B, Fuchs B, von Moos R, Schwitter M (2014) RANK ligand blockade with denosumab in combination with sorafenib in chemorefractory osteosarcoma: a possible step forward? Oncology 88(4):257–260
Endo-Munoz L, Cumming A, Rickwood D, Wilson D, Cueva C, Ng C, Strutton G, Cassady AI, Evdokiou A, Sommerville S, Dickinson I, Guminski A, Saunders NA (2010) Loss of osteoclasts contributes to development of osteosarcoma pulmonary metastases. Cancer Res 70(18):7063–7072
Endo-Munoz L, Evdokiou A, Evdokiou A, Saunders NA (2012) The role of osteoclasts and tumour-associated macrophages in osteosarcoma metastasis. Biochim Biophys Acta 1826(2):434–442
Matsuo K, Otaki N (2012) Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr 6(2):148–156
Fritsche-Guenther R, Noske A, Ungethum U, Kuban RJ, Schlag PM, Tunn PU, Karle J, Krenn V, Dietel M, Sers C (2010) De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology 57(6):836–850
Abdou AG, Abdel-Wahed MM, Asaad NY, Samaka RM, Abdallaha R (2010) Ephrin A4 expression in osteosarcoma, impact on prognosis, and patient outcome. Indian J Cancer 47(1):46–52
Varelias A, Koblar SA, Cowled PA, Carter CD, Clayer M (2002) Human osteosarcoma expresses specific ephrin profiles: implications for tumorigenicity and prognosis. Cancer 95(4):862–869
Garimella R, Washington L, Isaacson J, Vallejo J, Spence M, Tawfik O, Rowe P, Brotto M, Perez R (2014) Extracellular membrane vesicles derived from 143B osteosarcoma cells contain pro-osteoclastogenic cargo: a novel communication mechanism in osteosarcoma bone microenvironment. Transl Oncol 7(3):331–340
Yu L, Guo W, Zhao S, Wang F, Xu Y (2011) Fusion between cancer cells and myofibroblasts is involved in osteosarcoma. Oncol Lett 2(6):1083–1087
Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P (2013) Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta 1836(2):321–335
Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D (2008) Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68(11):4331–4339
Zhang L, Tang A, Zhou Y, Tang J, Luo Z, Jiang C, Li X, Xiang J, Li G (2012) Tumor-conditioned mesenchymal stem cells display hematopoietic differentiation and diminished influx of Ca2+. Stem Cells Dev 21(9):1418–1428
Hass R, Otte A (2012) Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun Signal 10(1):26
Rodriguez R, Rosu-Myles M, Arauzo-Bravo M, Horrillo A, Pan Q, Gonzalez-Rey E, Delgado M, Menendez P (2014) Human bone marrow stromal cells lose immunosuppressive and anti-inflammatory properties upon oncogenic transformation. Stem Cell Rep 3(4):606–619
Brune JC, Tormin A, Johansson MC, Rissler P, Brosjo O, Lofvenberg R, von Steyern FV, Mertens F, Rydholm A, Scheding S (2011) Mesenchymal stromal cells from primary osteosarcoma are non-malignant and strikingly similar to their bone marrow counterparts. Int J Cancer 129(2):319–330
Xu WT, Bian ZY, Fan QM, Li G, Tang TT (2009) Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett 281(1):32–41
Bian ZY, Fan QM, Li G, Xu WT, Tang TT (2010) Human mesenchymal stem cells promote growth of osteosarcoma: involvement of interleukin-6 in the interaction between human mesenchymal stem cells and Saos-2. Cancer Sci 101(12):2554–2560
Tu B, Du L, Fan QM, Tang Z, Tang TT (2012) STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett 325(1):80–88
Tu B, Peng ZX, Fan QM, Du L, Yan W, Tang TT (2014) Osteosarcoma cells promote the production of pro-tumor cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF-beta/Smad2/3 pathway. Exp Cell Res 320(1):164–173
Tsukamoto S, Honoki K, Fujii H, Tohma Y, Kido A, Mori T, Tsujiuchi T, Tanaka Y (2012) Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int J Oncol 40(1):163–169
Zhang P, Dong L, Long H, Yang TT, Zhou Y, Fan QY, Ma BA (2014) Homologous mesenchymal stem cells promote the emergence and growth of pulmonary metastases of the rat osteosarcoma cell line UMR-106. Oncol Lett 8(1):127–132
Kido A, Yoshitani K, Shimizu T, Akahane M, Fujii H, Tsukamoto S, Kondo Y, Honoki K, Imano M, Tanaka Y (2012) Effect of mesenchymal stem cells on hypoxia-induced desensitization of beta2-adrenergic receptors in rat osteosarcoma cells. Oncol Lett 4(4):745–750
Bonuccelli G, Avnet S, Grisendi G, Salerno M, Granchi D, Dominici M, Kusuzaki K, Baldini N (2014) Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget 5(17):7575–7588
Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K, Miyazawa K (2003) SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells. Cancer Res 63(22):7791–7798
Franchi A, Arganini L, Baroni G, Calzolari A, Capanna R, Campanacci D, Caldora P, Masi L, Brandi ML, Zampi G (1998) Expression of transforming growth factor beta isoforms in osteosarcoma variants: association of TGF beta 1 with high-grade osteosarcomas. J Pathol 185(3):284–289
Kloen P, Gebhardt MC, Perez-Atayde A, Rosenberg AE, Springfield DS, Gold LI, Mankin HJ (1997) Expression of transforming growth factor-beta (TGF-beta) isoforms in osteosarcomas: TGF-beta3 is related to disease progression. Cancer 80(12):2230–2239
Mohseny AB, Cai Y, Kuijjer M, Xiao W, van den Akker B, de Andrea CE, Jacobs R, ten Dijke P, Hogendoorn PC, Cleton-Jansen AM (2012) The activities of Smad and Gli mediated signalling pathways in high-grade conventional osteosarcoma. Eur J Cancer 48(18):3429–3438
Lamora A, Talbot J, Bougras G, Amiaud J, Leduc M, Chesneau J, Taurelle J, Stresing V, Le Deley MC, Heymann MF, Heymann D, Redini F, Verrecchia F (2014) Overexpression of smad7 blocks primary tumor growth and lung metastasis development in osteosarcoma. Clin Cancer Res 20(19):5097–5112
Yang RS, Wu CT, Lin KH, Hong RL, Liu TK, Lin KS (1998) Relation between histological intensity of transforming growth factor-beta isoforms in human osteosarcoma and the rate of lung metastasis. Tohoku J Exp Med 184(2):133–142
Xu S, Yang S, Sun G, Huang W, Zhang Y (2014) Transforming growth factor-beta polymorphisms and serum level in the development of osteosarcoma. DNA Cell Biol 33(11):802–806
Suzuki S, Kulkarni AB (2010) Extracellular heat shock protein HSP90beta secreted by MG63 osteosarcoma cells inhibits activation of latent TGF-beta1. Biochem Biophys Res Commun 398(3):525–531
Nguyen A, Scott MA, Dry SM, James AW (2014) Roles of bone morphogenetic protein signaling in osteosarcoma. Int Orthop 38(11):2313–2322
Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL, Sharff KA, He G, Bi Y, He BC, Bennett E, Huang J, Kang Q, Jiang W, Su Y, Zhu GH, Yin H, He Y, Wang Y, Souris JS, Chen L, Zuo GW, Montag AG, Reid RR, Haydon RC, Luu HH, He TC (2008) Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest 88(12):1264–1277
Sotobori T, Ueda T, Myoui A, Yoshioka K, Nakasaki M, Yoshikawa H, Itoh K (2006) Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin beta1 into lipid rafts. Exp Cell Res 312(19):3927–3938
Wang L, Park P, Zhang H, La Marca F, Claeson A, Valdivia J, Lin CY (2011) BMP-2 inhibits the tumorigenicity of cancer stem cells in human osteosarcoma OS99-1 cell line. Cancer Biol Ther 11(5):457–463
Lv Z, Wang C, Yuan T, Liu Y, Song T, Liu Y, Chen C, Yang M, Tang Z, Shi Q, Weng Y (2014) Bone morphogenetic protein 9 regulates tumor growth of osteosarcoma cells through the Wnt/beta-catenin pathway. Oncol Rep 31(2):989–994
Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ, Gitelis S, O’Keefe RJ, Konttinen YT, Yin G, Li TF (2013) Inhibition of the Wnt-beta-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun 431(2):274–279
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Zhang A, He S, Sun X, Ding L, Bao X, Wang N (2014) Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell Int 14(1):15
Kansara M, Teng MW, Smyth MJ, Thomas DM (2014) Translational biology of osteosarcoma. Nat Rev Cancer 14(11):722–735
Lin CH, Guo Y, Ghaffar S, McQueen P, Pourmorady J, Christ A, Rooney K, Ji T, Eskander R, Zi X, Hoang BH (2013) Dkk-3, a secreted wnt antagonist, suppresses tumorigenic potential and pulmonary metastasis in osteosarcoma. Sarcoma 2013:147541
Tian J, He H, Lei G (2014) Wnt/beta-catenin pathway in bone cancers. Tumour Biol 35(10):9439–9445
Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM (2010) Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol 220(1):24–33
Du X, Yang J, Yang D, Tian W, Zhu Z (2014) The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer 14:450
Krause U, Ryan DM, Clough BH, Gregory CA (2014) An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Dis 5:e1093
Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, Geller DS (2012) Cell surface receptor expression patterns in osteosarcoma. Cancer 118(3):740–749
Wiedłocha A, Falnes PO, Rapak A, Muñoz R, Klingenberg O, Olsnes S (1996) Stimulation of proliferation of a human osteosarcoma cell line by exogenous acidic fibroblast growth factor requires both activation of receptor tyrosine kinase and growth factor internalization. Mol Cell Biol 16(1):270–280
Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, Mansukhani A, Basilico C (2012) Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene 31(18):2270–2282
Shimizu T, Ishikawa T, Iwai S, Ueki A, Sugihara E, Onishi N, Kuninaka S, Miyamoto T, Toyama Y, Ijiri H, Mori H, Matsuzaki Y, Yaguchi T, Nishio H, Kawakami Y, Ikeda Y, Saya H (2012) Fibroblast growth factor-2 is an important factor that maintains cellular immaturity and contributes to aggressiveness of osteosarcoma. Mol Cancer Res 10(3):454–468
Tingting R, Wei G, Changliang P, Xinchang L, Yi Y (2010) Arsenic trioxide inhibits osteosarcoma cell invasiveness via MAPK signaling pathway. Cancer Biol Ther 10(3):251–257
Datsis GA, Berdiaki A, Nikitovic D, Mytilineou M, Katonis P, Karamanos NK, Tzanakakis GN (2011) Parathyroid hormone affects the fibroblast growth factor-proteoglycan signaling axis to regulate osteosarcoma cell migration. FEBS J 278(19):3782–3792
Pollak MN, Polychronakos C, Richard M (1990) Insulin like growth factor I: a potent mitogen for human osteogenic sarcoma. J Natl Cancer Inst 82(4):301–305
Jentzsch T, Robl B, Husmann M, Bode-Lesniewska B, Fuchs B (2014) Worse prognosis of osteosarcoma patients expressing IGF-1 on a tissue microarray. Anticancer Res 34(8):3881–3889
Pollak M, Sem AW, Richard M, Tetenes E, Bell R (1992) Inhibition of metastatic behavior of murine osteosarcoma by hypophysectomy. J Natl Cancer Inst 84(12):966–971
Chou AJ, Geller DS, Gorlick R (2008) Therapy for osteosarcoma: where do we go from here? Paediatr Drugs 10(5):315–327
Cao Y, Roth M, Piperdi S, Montoya K, Sowers R, Rao P, Geller D, Houghton P, Kolb EA, Gill J, Gorlick R (2014) Insulin-like growth factor 1 receptor and response to anti-IGF1R antibody therapy in osteosarcoma. PLoS One 9(8):e106249
Chen D, Zhang YJ, Zhu KW, Wang WC (2013) A systematic review of vascular endothelial growth factor expression as a biomarker of prognosis in patients with osteosarcoma. Tumour Biol 34(3):1895–1899
Ohba T, Cates JM, Cole HA, Slosky DA, Haro H, Ando T, Schwartz HS, Schoenecker JG (2014) Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res 12(8):1100–1111
Cho HJ, Lee TS, Park JB, Park KK, Choe JY, Sin DI, Park YY, Moon YS, Lee KG, Yeo JH, Han SM, Cho YS, Choi MR, Park NG, Lee YS, Chang YC (2007) Disulfiram suppresses invasive ability of osteosarcoma cells via the inhibition of MMP-2 and MMP-9 expression. J Biochem Mol Biol 40(6):1069–1076
Mohseny AB, Xiao W, Carvalho R, Spaink HP, Hogendoorn PC, Cleton-Jansen AM (2012) An osteosarcoma zebrafish model implicates Mmp-19 and Ets-1 as well as reduced host immune response in angiogenesis and migration. J Pathol 227(2):245–253
Kang HG, Kim HS, Kim KJ, Oh JH, Lee MR, Seol SM, Han I (2007) RECK expression in osteosarcoma: correlation with matrix metalloproteinases activation and tumor invasiveness. J Orthop Res 25(5):696–702
de Nigris F, Mancini FP, Schiano C, Infante T, Zullo A, Minucci PB, Al-Omran M, Giordano A, Napoli C (2013) Osteosarcoma cells induce endothelial cell proliferation during neo-angiogenesis. J Cell Physiol 228(4):846–852
Ren K, Yao N, Wang G, Tian L, Ma J, Shi X, Zhang L, Zhang J, Zhou X, Zhou G, Wu S, Sun X (2014) Vasculogenic mimicry: a new prognostic sign of human osteosarcoma. Hum Pathol 45(10):2120–2129
Sampson VB, Gorlick R, Kamara D, Anders Kolb E (2013) A review of targeted therapies evaluated by the pediatric preclinical testing program for osteosarcoma. Front Oncol 3:132
Sulzbacher I, Birner P, Trieb K, Träxler M, Lang S, Chott A (2003) Expression of platelet-derived growth factor-AA is associated with tumor progression in osteosarcoma. Mod Pathol 16(1):66–71
Takagi S, Takemoto A, Takami M, Oh-Hara T, Fujita N (2014) Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci 105(8):983–988
Lo WW, Pinnaduwage D, Gokgoz N, Wunder JS, Andrulis IL (2014) Aberrant hedgehog signaling and clinical outcome in osteosarcoma. Sarcoma 2014:261804
Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK (2014) Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 33(40):4857–4866
Zhang YH, Li B, Shen L, Shen Y, Chen XD (2013) The role and clinical significance of YES-associated protein 1 in human osteosarcoma. Int J Immunopathol Pharmacol 26(1):157–167
Tao J, Jiang MM, Jiang L, Salvo JS, Zeng HC, Dawson B, Bertin TK, Rao PH, Chen R, Donehower LA, Gannon F, Lee BH (2014) Notch activation as a driver of osteogenic sarcoma. Cancer Cell 26(3):390–401
Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, Donehower LA, Lee B (2009) Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet 18(8):1464–1470
Hughes DP (2009) How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res 152:479–496
Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, Komiya S (2009) Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer 100(12):1957–1965
Kafchinski LA, Jones KB (2014) MicroRNAs in osteosarcomagenesis. Adv Exp Med Biol 804:119–127
Nugent M (2014) MicroRNA function and dysregulation in bone tumors: the evidence to date. Cancer Manag Res 6:15–25
Sarver AL, Thayanithy V, Scott MC, Cleton-Jansen AM, Hogendoorn PC, Modiano JF, Subramanian S (2013) MicroRNAs at the human 14q32 locus have prognostic significance in osteosarcoma. Orphanet J Rare Dis 8:7
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75(5):843–854
Gougelet A, Pissaloux D, Besse A, Perez J, Duc A, Dutour A, Blay JY, Alberti L (2011) Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int J Cancer 129(3):680–690
Arabi L, Gsponer JR, Smida J, Nathrath M, Perrina V, Jundt G, Ruiz C, Quagliata L, Baumhoer D (2014) Upregulation of the miR-17-92 cluster and its two paraloga in osteosarcoma—reasons and consequences. Genes Cancer 5(1–2):56–63
Zhao H, Guo M, Zhao G, Ma Q, Ma B, Qiu X, Fan Q (2012) miR-183 inhibits the metastasis of osteosarcoma via downregulation of the expression of Ezrin in F5M2 cells. Int J Mol Med 30(5):1013–1020
Zhou X, Wei M, Wang W (2013) MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Biophys Res Commun 437(4):653–658
Poos K, Smida J, Nathrath M, Maugg D, Baumhoer D, Korsching E (2013) How microRNA and transcription factor co-regulatory networks affect osteosarcoma cell proliferation. PLoS Comput Biol 9(8):e1003210
Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E (2014) Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl Res 164(5):359–365
Kansara M, Leong HS, Lin DM, Popkiss S, Pang P, Garsed DW, Walkley CR, Cullinane C, Ellul J, Haynes NM, Hicks R, Kuijjer ML, Cleton-Jansen AM, Hinds PW, Smyth MJ, Thomas DM (2013) Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J Clin Invest 123(12):5351–5360
Wang L, Zhang Q, Chen W, Shan B, Ding Y, Zhang G, Cao N, Liu L, Zhang Y (2013) B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One 8(8):e70689
Wang M, Wang L, Ren T, Xu L, Wen Z (2013) IL-17A/IL-17RA interaction promoted metastasis of osteosarcoma cells. Cancer Biol Ther 14(2):155–163
Moore C, Eslin D, Levy A, Roberson J, Giusti V, Sutphin R (2010) Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatr Blood Cancer 55(6):1096–1102
Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A (2007) Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol 14(10):2887–2895
Kawano M, Itonaga I, Iwasaki T, Tsuchiya H, Tsumura H (2012) Anti-TGF-beta antibody combined with dendritic cells produce antitumor effects in osteosarcoma. Clin Orthop Relat Res 470(8):2288–2294
DeRenzo C, Gottschalk S (2014) Genetically modified T-cell therapy for osteosarcoma. Adv Exp Med Biol 804:323–340
Rainusso N, Brawley VS, Ghazi A, Hicks MJ, Gottschalk S, Rosen JM, Ahmed N (2012) Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther 19(3):212–217
Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H (2005) Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 23(9):2004–2011
von Luettichau I, Segerer S, Wechselberger A, Notohamiprodjo M, Nathrath M, Kremer M, Henger A, Djafarzadeh R, Burdach S, Huss R, Nelson PJ (2008) A complex pattern of chemokine receptor expression is seen in osteosarcoma. BMC Cancer 8:23
Wang SW, Wu HH, Liu SC, Wang PC, Ou WC, Chou WY, Shen YS, Tang CH (2012) CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PLoS One 7(4):e35101
Wang SW, Liu SC, Sun HL, Huang TY, Chan CH, Yang CY, Yeh HI, Huang YL, Chou WY, Lin YM, Tang CH (2015) CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 36(1):104–114
Chen PC, Cheng HC, Yang SF, Lin CW, Tang CH (2014) The CCN family proteins: modulators of bone development and novel targets in bone-associated tumors. Biomed Res Int 2014:437096
Manara MC, Perbal B, Benini S, Strammiello R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami J, Yeger H, Picci P, Scotlandi K (2002) The expression of ccn3(nov) gene in musculoskeletal tumors. Am J Pathol 160(3):849–859
Sabile AA, Arlt MJ, Muff R, Bode B, Langsam B, Bertz J, Jentzsch T, Puskas GJ, Born W, Fuchs B (2012) Cyr61 expression in osteosarcoma indicates poor prognosis and promotes intratibial growth and lung metastasis in mice. J Bone Miner Res 27(1):58–67
Chen PC, Cheng HC, Tang CH (2013) CCN3 promotes prostate cancer bone metastasis by modulating the tumor-bone microenvironment through RANKL-dependent pathway. Carcinogenesis 34(7):1669–1679
Zhu L, McManus MM, Hughes DP (2013) Understanding the biology of bone sarcoma from early initiating events through late events in metastasis and disease progression. Front Oncol 3:230
Ren L, Khanna C (2014) Role of ezrin in osteosarcoma metastasis. Adv Exp Med Biol 804:181–201
Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10(2):182–186
Ren L, Hong SH, Cassavaugh J, Osborne T, Chou AJ, Kim SY, Gorlick R, Hewitt SM, Khanna C (2009) The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene 28(6):792–802
Koshkina NV, Khanna C, Mendoza A, Guan H, DeLauter L, Kleinerman ES (2007) Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res 5(10):991–999
Gordon N, Arndt CA, Hawkins DS, Doherty DK, Inwards CY, Munsell MF, Stewart J, Koshkina NV, Kleinerman ES (2005) Fas expression in lung metastasis from osteosarcoma patients. J Pediatr Hematol Oncol 27(11):611–615
Huang G, Nishimoto K, Yang Y, Kleinerman ES (2014) Participation of the Fas/FasL signaling pathway and the lung microenvironment in the development of osteosarcoma lung metastases. Adv Exp Med Biol 804:203–217
Rao-Bindal K, Zhou Z, Kleinerman ES (2012) MS-275 sensitizes osteosarcoma cells to Fas ligand-induced cell death by increasing the localization of Fas in membrane lipid rafts. Cell Death Dis 3:e369
Hou CH, Lin FL, Tong KB, Hou SM, Liu JF (2014) Transforming growth factor alpha promotes osteosarcoma metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem Pharmacol 89(4):453–463
El Naggar A, Clarkson P, Zhang F, Mathers J, Tognon C, Sorensen PH (2012) Expression and stability of hypoxia inducible factor 1alpha in osteosarcoma. Pediatr Blood Cancer 59(7):1215–1222
Guo M, Cai C, Zhao G, Qiu X, Zhao H, Ma Q, Tian L, Li X, Hu Y, Liao B, Ma B, Fan Q (2014) Hypoxia promotes migration and induces CXCR4 expression via HIF-1alpha activation in human osteosarcoma. PLoS One 9(3):e90518
Scholten DJ 2nd, Timmer CM, Peacock JD, Pelle DW, Williams BO, Steensma MR (2014) Down regulation of wnt signaling mitigates hypoxia-induced chemoresistance in human osteosarcoma cells. PLoS One 9(10):e111431
Adamski J, Price A, Dive C, Makin G (2013) Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of HIF-1Alpha. PLoS One 8(6):e65304
Roncuzzi L, Pancotti F, Baldini N (2014) Involvement of HIF-1alpha activation in the doxorubicin resistance of human osteosarcoma cells. Oncol Rep 32(1):389–394
Harada R, Kawamoto T, Ueha T, Minoda M, Toda M, Onishi Y, Fukase N, Hara H, Sakai Y, Miwa M, Kuroda R, Kurosaka M, Akisue T (2013) Reoxygenation using a novel CO2 therapy decreases the metastatic potential of osteosarcoma cells. Exp Cell Res 319(13):1988–1997
Matsubara T, Diresta GR, Kakunaga S, Li D, Healey JH (2013) additive influence of extracellular ph, oxygen tension, and pressure on invasiveness and survival of human osteosarcoma cells. Front Oncol 3:199
Rochet N, Loubat A, Laugier JP, Hofman P, Bouler JM, Daculsi G, Carle GF, Rossi B (2003) Modification of gene expression induced in human osteogenic and osteosarcoma cells by culture on a biphasic calcium phosphate bone substitute. Bone 32(6):602–610
Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, Iwakuma T (2010) CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res 70(11):4602–4612
Basu-Roy U, Basilico C, Mansukhani A (2013) Perspectives on cancer stem cells in osteosarcoma. Cancer Lett 338(1):158–167
Siclari VA, Qin L (2010) Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res 5:78
Zhang H, Wu H, Zheng J, Yu P, Xu L, Jiang P, Gao J, Wang H, Zhang Y (2013) Transforming growth factor beta1 signal is crucial for dedifferentiation of cancer cells to cancer stem cells in osteosarcoma. Stem Cells 31(3):433–446
Wang L, Park P, Zhang H, La Marca F, Lin CY (2011) Prospective identification of tumorigenic osteosarcoma cancer stem cells in OS99-1 cells based on high aldehyde dehydrogenase activity. Int J Cancer 128(2):294–303
Acknowledgments
We thank Dr. Ashley Hamilton (from The Francis Crick Institute, London, UK) for her comprehensive revision of the manuscript. This work was supported by the Plan Nacional de I+D+i 2008–2011 [ISCIII/FEDER (PI11/00377, Miguel Servet Program CP11/00024 & CP11/00206) and RTICC (RD12/0036/0015, RD12/0036/0027 & RD12/0036/0017)], the Plan Nacional de I+D+i 2013–2016 [MINECO/FEDER (SAF-2013-42946-R & SAF2013-43065)], Grupo Español de Investigación en Sarcomas (GEIS), Generalitat de Catalunya (Grupo SGR330), Health Canada and Obra Social La Caixa/Fundaciò Josep Carreras.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Alfranca, L. Martinez-Cruzado and J. Tornin contributed equally to this article.
Rights and permissions
About this article
Cite this article
Alfranca, A., Martinez-Cruzado, L., Tornin, J. et al. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 72, 3097–3113 (2015). https://doi.org/10.1007/s00018-015-1918-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1918-y