Abstract
Objective
Inflammation and proliferation of vascular smooth muscle cells (VSMCs), induced by angiotensin II (AngII) and other growth factors, play important roles in the pathogenesis of hypertension, restenosis, and atherosclerosis. Dihydroartemisinin (DHA) exhibits broad protective effects. However, the effects of DHA on AngII-induced inflammation and proliferation of VSMCs remain unknown.
Materials and methods
AngII was used to construct VSMCs and vascular inflammation model in vitro and in vivo. The protective roles of DHA in inflammatory response and proliferation were evaluated through CCK-8, BrdU assay and immunofluorescence staining. The level of mRNA N6-methyladenosine was measured by m6A-RNA immunoprecipitation (MeRIP) assay. Western blot and quantitative real-time PCR were used to investigate the relationship between FTO and its potential downstream signaling molecules.
Results
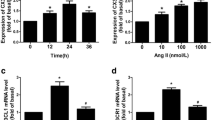
In the present study, we found that DHA significantly suppressed AngII-induced proliferation of VSMCs and the expression of IL-6 and Ccl2 in a dose-dependent manner. Additionally, we confirmed that fat mass and obesity-associated (FTO) plays a critical role in AngII-induced VSMC proliferation and inflammation. FTO knockdown increased the methylation level of NR4A3 mRNA, whereas FTO, but not mutated FTO overexpression, reduced the methylation level of NR4A3 mRNA. These results suggest that DHA plays a protective role in AngII-induced VSMC proliferation and the associated inflammation by inhibiting the FTO/NR4A3 axis.
Conclusion
Our findings provide new insight into the mechanisms of DHA and its critical role in the pathogenesis of hypertension-related vascular complications.






Similar content being viewed by others
References
Touyz RM, Alves-Lopes R, Rios FJ, et al. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114:529–39.
Patel S, Rauf A, Khan H, et al. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–25.
Das S, Zhang E, Senapati P, et al. A novel angiotensin II-induced long noncoding RNA giver regulates oxidative stress, inflammation, and proliferation in vascular smooth muscle cells. Circ Res. 2018;123:1298–312.
Yang Z, Zheng B, Zhang Y, et al. miR-155-dependent regulation of mammalian sterile 20-like kinase 2 (MST2) coordinates inflammation, oxidative stress and proliferation in vascular smooth muscle cells. Biochim Biophys Acta. 2015;1852:1477–89.
Chen M, Wei L, Law CT, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–70.
Li Z, Weng H, Su R, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–41.
Mizuno TM. Fat mass and obesity associated (FTO) Gene and Hepatic Glucose and Lipid Metabolism. Nutrients 2018, 10.
Rong ZX, Li Z, He JJ, et al. Downregulation of fat mass and obesity associated (FTO) promotes the progression of intrahepatic cholangiocarcinoma. Front Oncol. 2019;9:369.
Yajnik CS, Janipalli CS, Bhaskar S, et al. FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia. 2009;52:247–52.
Sun L, Ma L, Zhang H, et al. Fto Deficiency reduces anxiety- and depression-like behaviors in mice via alterations in gut microbiota. Theranostics. 2019;9:721–33.
Wei J, Liu F, Lu Z, et al. Differential m(6)A, m(6)Am, and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol Cell 2018,71:973–985 e975.
Kang H, Zhang Z, Yu L, et al. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J Cell Biochem. 2018;119:5676–85.
Zhao X, Yang Y, Sun BF, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–19.
Zou D, Dong L, Li C, et al. The m(6)A eraser FTO facilitates proliferation and migration of human cervical cancer cells. Cancer Cell Int. 2019;19:321.
Zhu B, Gong Y, Shen L, et al. Total Panax notoginseng saponin inhibits vascular smooth muscle cell proliferation and migration and intimal hyperplasia by regulating WTAP/p16 signals via m(6)A modulation. Biomed Pharmacother 2020,124:109935.
D’Alessandro S, Basilico N, Corbett Y, et al. Hypoxia modulates the effect of dihydroartemisinin on endothelial cells. Biochem Pharmacol. 2011;82:476–84.
Wu GD, Zhou HJ, Wu XH. Apoptosis of human umbilical vein endothelial cells induced by artesunate. Vascul Pharmacol. 2004;41:205–12.
Chen HH, Zhou HJ, Fang X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol Res. 2003;48:231–6.
Okabe S, Higaki E, Higuchi T, et al. Biochemical and pharmacological analysis of 2-[(2-dimethylaminobenzyl)sulfinyl] benzimidazole (NC-1300), a new proton pump inhibitor. Jpn J Pharmacol. 1986;40:239–49.
Zhou HJ, Wang WQ, Wu GD, et al. Artesunate inhibits angiogenesis and downregulates vascular endothelial growth factor expression in chronic myeloid leukemia K562 cells. Vascul Pharmacol. 2007;47:131–8.
Dong F, Zhou X, Li C, et al. Dihydroartemisinin targets VEGFR2 via the NF-kappaB pathway in endothelial cells to inhibit angiogenesis. Cancer Biol Ther. 2014;15:1479–88.
Zhang XH, Zheng B, Yang Z, et al. TMEM16A and myocardin form a positive feedback loop that is disrupted by KLF5 during Ang II-induced vascular remodeling. Hypertension. 2015;66:412–21.
Li N, Sun W, Zhou X, et al. Dihydroartemisinin protects against dextran sulfate sodium-induced colitis in mice through inhibiting the PI3K/AKT and NF-kappaB signaling pathways. Biomed Res Int. 2019;2019:1415809.
Zhang F, Ma Q, Xu Z, et al. Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer Res. 2017;36:68.
Li Y, Wang Y, Kong R, et al. Dihydroartemisinin suppresses pancreatic cancer cells via a microRNA-mRNA regulatory network. Oncotarget. 2016;7:62460–73.
Yang Z, Qu CB, Zhang Y, et al. Dysregulation of p53-RBM25-mediated circAMOTL1L biogenesis contributes to prostate cancer progression through the circAMOTL1L-miR-193a-5p-Pcdha pathway. Oncogene. 2019;38:2516–32.
Li J, Han Y, Zhang H, et al. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479–85.
Su R, Dong L, Li C, et al. R-2HG Exhibits Anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell 2018, 172:90–105 e123.
Ma D, Liu X, Zhang JJ, et al. Vascular Smooth Muscle FTO Promotes Aortic Dissecting Aneurysms via m6A Modification of Klf5. Front Cardiovasc Med 2020, 7:592550.
Munoz-Durango N, Fuentes CA, Castillo AE, et al. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci 2016, 17.
Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–74.
Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–74.
Ruiz-Ortega M, Esteban V, Ruperez M, et al. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens. 2006;15:159–66.
Bookout AL, Jeong Y, Downes M, et al. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–99.
Mullican SE, Zhang S, Konopleva M, et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730–5.
Chao LC, Wroblewski K, Zhang Z, et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58:2788–96.
Pearen MA, Myers SA, Raichur S, et al. The orphan nuclear receptor, NOR-1, a target of beta-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology. 2008;149:2853–65.
Tessem JS, Moss LG, Chao LC, et al. Nkx6.1 regulates islet beta-cell proliferation via Nr4a1 and Nr4a3 nuclear receptors. Proc Natl Acad Sci U S A 2014, 111:5242–5247.
Myers SA, Eriksson N, Burow R, et al. Beta-adrenergic signaling regulates NR4A nuclear receptor and metabolic gene expression in multiple tissues. Mol Cell Endocrinol. 2009;309:101–8.
Wilson TE, Fahrner TJ, Johnston M, et al. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–300.
Herring JA, Elison WS, Tessem JS. Function of Nr4a orphan nuclear receptors in proliferation, apoptosis and fuel utilization across tissues. Cells 2019, 8.
Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol. 2010;30:1535–41.
Yang B, Gao X, Sun Y, et al. Dihydroartemisinin alleviates high glucose-induced vascular smooth muscle cells proliferation and inflammation by depressing the miR-376b-3p/KLF15 pathway. Biochem Biophys Res Commun. 2020;530:574–80.
Yin J, Xia W, Zhang Y, et al. Role of dihydroartemisinin in regulating prostaglandin E2 synthesis cascade and inflammation in endothelial cells. Heart Vessels. 2018;33:1411–22.
Liu X, Lu J, Liao Y, et al. Dihydroartemisinin attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation and oxidative stress. Biomed Pharmacother 2019, 117:109070.
Xu X, Jiang R, Chen M, et al. Puerarin decreases collagen secretion in angii-induced atrial fibroblasts through inhibiting autophagy via the JNK-Akt-mTOR signaling pathway. J Cardiovasc Pharmacol. 2019;73:373–82.
Chen BC, Shibu MA, Kuo CH, et al. E4BP4 inhibits AngII-induced apoptosis in H9c2 cardiomyoblasts by activating the PI3K-Akt pathway and promoting calcium uptake. Exp Cell Res. 2018;363:227–34.
Wang JY, Chen LJ, Qiang P. The Potential role of N6-methyladenosine (m6A) demethylase fat mass and obesity-associated gene (FTO) in human cancers. Onco Targets Ther. 2020;13:12845–56.
Liu J, Ren Y, Hou Y, et al. Dihydroartemisinin induces endothelial cell autophagy through suppression of the Akt/mTOR pathway. J Cancer. 2019;10:6057–64.
Acknowledgements
This study was partially supported by The Natural Science Foundation of Hebei Province (No. H2019206191 and H2021206161); This study was also supported by The government-funded clinical medicine outstanding talent training project (303-16-20-11) and High-level Talent Funding Project in Hebei Province (A202101059).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Additional information
Communicated by Andrew Roberts.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huo, YB., Gao, X., Peng, Q. et al. Dihydroartemisinin alleviates AngII-induced vascular smooth muscle cell proliferation and inflammatory response by blocking the FTO/NR4A3 axis. Inflamm. Res. 71, 243–253 (2022). https://doi.org/10.1007/s00011-021-01533-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01533-3