Abstract
Objective
Microglia/macrophage activation is previously reported to be involved in various ocular diseases. However, the separate role of M1/M2 phenotype microglia/macrophage in the pathological process of oxygen-induced retinopathy (OIR) remains unknown. In this research, we explored the role and regulatory mechanism of M1/M2 microglia/macrophage in OIR in C57BL/6J mice. Furthermore, we demonstrated the time phase of M1/M2 shifting of microglia/macrophage during the natural process of OIR, which is very essential for further investigations.
Materials and methods
C57BL/6j pups were exposed to hyperoxia environment from postnatal 7(P7) to P12 then returned to normoxia. The mice were then euthanized, and the eyes were harvested at a series of time points for further investigation. The M1/M2 phenotype microglia/macrophage activity was presented by immunofluorescent staining and real-time quantitative polymerase chain reaction (qPCR). The NF-κb-STAT3 signaling and IL-4-STAT6-PPAR-γ signaling pathway activity was examined by western blot analysis.
Results
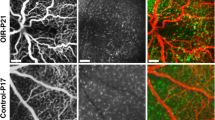
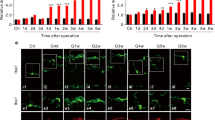
The microglia/macrophage were activated when the OIR model was set up after P12. The M1 microglia/macrophage activation was found in neovascularization (NV) tufts in both central and peripheral retina, which started from P12 when the mice were returned to normoxia environment and peaked at P17. During this period of time, the NF-κb-STAT3 signaling pathway was activated, resulting in the upregulated M1 phenotype microglia/macrophage polarization, along with the enhanced inflammatory cytokine expression including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β. Consequently, the NV tufts were observed from P12 and the volume continued to increase until P17. However, the M2 phenotype microglia/macrophage activity took over during the late phase of OIR started from P17. The IL-4-STAT6-PPAR-γ signaling activity was upregulated from P17 and peaked at P20, inducing M2 phenotype microglia polarization, which consequently led to the inhibition of inflammatory cytokines and spontaneous regression of NV tufts.
Conclusions
Microglia/macrophage participate actively in the natural process of OIR in mice, and two phenotypes exert different functions. Treatment modulating microglia/macrophage polarize toward M2 phenotype might be a novel and promising method for ocular neovascular diseases such as retinopathy of prematurity (ROP), wet age-related macular degeneration (wAMD), and diabetic retinopathy (DR).






Similar content being viewed by others
References
Smith L, Wesolowski E, McLellan A, Kostyk S, D’Amato R, Sullivan R, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–11.
Villacampa P, Menger K, Abelleira L, Ribeiro J, Duran Y, Smith A, et al. Accelerated oxygen-induced retinopathy is a reliable model of ischemia-induced retinal neovascularization. PLoS ONE. 2017;12(6):e0179759. https://doi.org/10.1371/journal.pone.0179759.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (New York, NY). 2010;330(6005):841–5. https://doi.org/10.1126/science.1194637.
Rathnasamy G, Foulds W, Ling E, Kaur C. Retinal microglia—a key player in healthy and diseased retina. Prog Neurobiol. 2019;173:18–40. https://doi.org/10.1016/j.pneurobio.2018.05.006.
Connor K, SanGiovanni J, Lofqvist C, Aderman C, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–73. https://doi.org/10.1038/nm1591.
Okunuki Y, Mukai R, Pearsall E, Klokman G, Husain D, Park D, et al. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc Natl Acad Sci USA. 2018;115(27):E6264–73. https://doi.org/10.1073/pnas.1719601115.
Ma W, Silverman S, Zhao L, Villasmil R, Campos M, Amaral J, et al. Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. eLife. 2019. https://doi.org/10.7554/eLife.42049.
Dagkalis A, Wallace C, Hing B, Liversidge J, Crane I. CX3CR1-deficiency is associated with increased severity of disease in experimental autoimmune uveitis. Immunology. 2009;128(1):25–33. https://doi.org/10.1111/j.1365-2567.2009.03046.x.
Dong H, Zhang X, Wang Y, Zhou X, Qian Y, Zhang S. Suppression of brain mast cells degranulation inhibits microglial activation and central nervous system inflammation. Mol Neurobiol. 2017;54(2):997–1007. https://doi.org/10.1007/s12035-016-9720-x.
Mantovani A, Biswas S, Galdiero M, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85. https://doi.org/10.1002/path.4133.
Szabo M, Gulya K. Development of the microglial phenotype in culture. Neuroscience. 2013;241:280–95. https://doi.org/10.1016/j.neuroscience.2013.03.033.
Martinez F, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. https://doi.org/10.1146/annurev.immunol.021908.132532.
Fischer F, Martin G, Agostini H. Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. J Neuroinflamm. 2011;8:120. https://doi.org/10.1186/1742-2094-8-120.
Xu Y, Lu X, Hu Y, Yang B, Tsui C, Yu S, et al. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res. 2018;64(4):e12473. https://doi.org/10.1111/jpi.12473.
Yang B, Xu Y, Yu S, Huang Y, Lu L, Liang X. Anti-angiogenic and anti-inflammatory effect of Magnolol in the oxygen-induced retinopathy model. Inflamm Res. 2016;65(1):81–93. https://doi.org/10.1007/s00011-015-0894-x.
Haage V, Semtner M, Vidal R, Hernandez D, Pong W, Chen Z, et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol Commun. 2019;7(1):20. https://doi.org/10.1186/s40478-019-0665-y.
Xu Y, Cui K, Li J, Tang X, Lin J, Lu X, et al. Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. J Pineal Res. 2020. https://doi.org/10.1111/jpi.12660.
Ransohoff R, Perry V. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. https://doi.org/10.1146/annurev.immunol.021908.132528.
Bosco A, Steele M, Vetter M. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519(4):599–620. https://doi.org/10.1002/cne.22516.
Zhang L, Cui X, Jauregui R, Park K, Justus S, Tsai Y, et al. Genetic rescue reverses microglial activation in preclinical models of retinitis pigmentosa. Mol Ther. 2018;26(8):1953–64. https://doi.org/10.1016/j.ymthe.2018.06.014.
Telegina D, Kozhevnikova O, Kolosova N. Changes in retinal glial cells with age and during development of age-related macular degeneration. Biochemistry. 2018;83(9):1009–17. https://doi.org/10.1134/s000629791809002x.
Gallego B, Salazar J, de Hoz R, Rojas B, Ramírez A, Salinas-Navarro M, et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflamm. 2012;9:92. https://doi.org/10.1186/1742-2094-9-92.
Brandenburg S, Müller A, Turkowski K, Radev Y, Rot S, Schmidt C, et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016;131(3):365–78. https://doi.org/10.1007/s00401-015-1529-6.
Takeda A, Shinozaki Y, Kashiwagi K, Ohno N, Eto K, Wake H, et al. Microglia mediate non-cell-autonomous cell death of retinal ganglion cells. Glia. 2018;66(11):2366–84. https://doi.org/10.1002/glia.23475.
David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–99. https://doi.org/10.1038/nrn3053.
Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210:3–12. https://doi.org/10.1016/j.jneuroim.2009.02.003.
Lange C, Ehlken C, Stahl A, Martin G, Hansen L, Agostini H. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefe’s Arch Clin Exp Ophthal. 2009;247(9):1205–11. https://doi.org/10.1007/s00417-009-1116-4.
Stahl A, Chen J, Sapieha P, Seaward M, Krah N, Dennison R, et al. Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy. Am J Pathol. 2010;177(6):2715–23. https://doi.org/10.2353/ajpath.2010.100526.
Han R, Xiao J, Zhai H, Hao J. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J Neuroinflamm. 2016;13(1):97. https://doi.org/10.1186/s12974-016-0559-x.
Zhao Q, Wu X, Yan S, Xie X, Fan Y, Zhang J, et al. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. J Neuroinflamm. 2016;13(1):259. https://doi.org/10.1186/s12974-016-0728-y.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. https://doi.org/10.1038/nrc2734.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. https://doi.org/10.1038/nature07205.
Bollrath J, Phesse T, von Burstin V, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. https://doi.org/10.1016/j.ccr.2009.01.002.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu G, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13. https://doi.org/10.1016/j.ccr.2009.01.001.
Yin Z, Ma T, Lin Y, Lu X, Zhang C, Chen S, et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9419–32. https://doi.org/10.1002/jcb.27259.
Zhong Z, Wen Z, Darnell J. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science (New York, NY). 1994;264(5155):95–8. https://doi.org/10.1126/science.8140422.
Oh H, Park S, Kang M, Kim Y, Lee E, Kim D, et al. Asaronic acid attenuates macrophage activation toward M1 phenotype through inhibition of NF-κB pathway and JAK-STAT signaling in glucose-loaded murine macrophages. J Agric Food Chem. 2019;67(36):10069–78. https://doi.org/10.1021/acs.jafc.9b03926.
Egholm C, Heeb L, Impellizzieri D, Boyman O. The Regulatory effects of interleukin-4 receptor signaling on neutrophils in type 2 immune responses. Front Immunol. 2019;10:2507. https://doi.org/10.3389/fimmu.2019.02507.
Zheng H, Wu X, Wu D, Jiang R, Castillo E, Chock C, et al. Treg expression of CIS suppresses allergic airway inflammation through antagonizing an autonomous TH2 program. Mucosal Immunol. 2020;13(2):293–302. https://doi.org/10.1038/s41385-019-0236-3.
Balce D, Li B, Allan E, Rybicka J, Krohn R, Yates R. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood. 2011;118(15):4199–208. https://doi.org/10.1182/blood-2011-01-328906.
Luo Y, Yin W, Signore A, Zhang F, Hong Z, Wang S, et al. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97(2):435–48. https://doi.org/10.1111/j.1471-4159.2006.03758.x.
Glass C, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol. 2016;17(1):26–33. https://doi.org/10.1038/ni.3306.
Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, et al. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis. 2014;71:280–91. https://doi.org/10.1016/j.nbd.2014.08.011.
Daniel B, Nagy G, Horvath A, Czimmerer Z, Cuaranta-Monroy I, Poliska S, et al. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 2018;46(9):4425–39. https://doi.org/10.1093/nar/gky157.
Acknowledgements
This research is supported by the national natural science foundation of China (No. 81870668), and the Young Scientist Fund of the National Natural Science Foundation of China (No. 81900864).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that none of the authors have any financial or scientific conflicts of interest with regard to the research described in this manuscript.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Yu, S., Lu, X. et al. The phase changes of M1/M2 phenotype of microglia/macrophage following oxygen-induced retinopathy in mice. Inflamm. Res. 70, 183–192 (2021). https://doi.org/10.1007/s00011-020-01427-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01427-w